Organic Chem TYS questions
-
1. I have this particular query to ask of you guys.
Firstly, why is the structure below considered an alkene with the molecular formula C4H8?
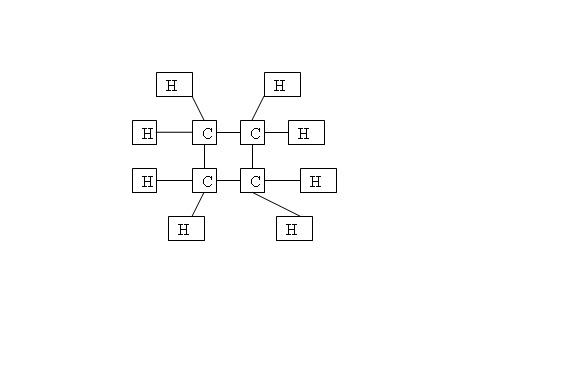
Though alkene is supposed to have C=C double bond? Anyway, this is one of the options chosen in the answer. Thanks all.
-
This structure is a cycloalkane or more accurately cyclobutane. Even though this has the same general formula as an alkene, this is not an alkene.
Did the answer say that this is an alkene?
-
Yea. The question asked "Which structures represent alkenes with the molecular structure C4H8?"
This was included in the answer scheme.
-
2. Can someone tell me why this is considered an addition reaction
nC2H4 ---> --------(CH2-------CH2)--------n
-
1) I will never consider a cycloalkane an alkene since they have very different chemical reactions but a cyclobutane is very strain and does break easily and react. Unless all the other choices in the question don't even have the alkene general formula, i will never take that as the answer.
2) It is consider an additional reaction since each molecule is added together to form a chain by the breaking of the double bond, there is no additional product formed through this reaction.
-