Electrolysis
-
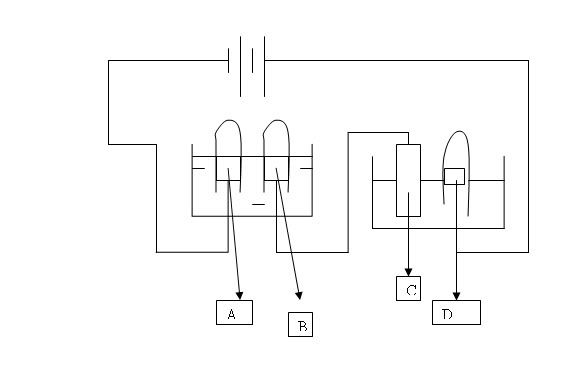
Just a short question
After electrolysis for 15 minutes, 140 cm3 of gas was collected in the test tube at electrode A. 120 cm3 of chlorine gas was collected at D instead of 140cm3 that was expected. Explain.
-
Originally posted by bonkysleuth:

Just a short question
After electrolysis for 15 minutes, 140 cm3 of gas was collected in the test tube at electrode A. 120 cm3 of chlorine gas was collected at D instead of 140cm3 that was expected. Explain.
The info given by the question is incomplete, thus no answer can be definite.Notwithstanding, nevertheless, regardless, it is common enough that volumes of gases collected over electrodes do not fit theoretical volumes. Reasons include :
- Gases undergoing hydrolysis, eg. chlorine + water --> chloric(I) acid (latin name : hypochlorous acid) + hydrochloric acid
- Graphite anodes reacting with the oxygen produced at the anode, to form carbon monoxide gas (carbon dioxide gas would not be an acceptable answer, based on stoichiometry).
- etc.
-
i believe 1 more is chlorine more soluble in warm temp.
-
yes......