BedokFunland JC's A Level H2 Chemistry Qns (Part 2)
-
'A' Level H2 Chemistry
Topic : Solubility (Ionic) Equilibria
Given that Ksp of silver sulfate is 1.4 x 10^-5, what is the sample mass of solid silver nitrate to be added to
250cm3 of saturated silver sulfate solution, such that the mass solubility of silver sulfate is reduced to 0.3121 g/dm3?Solution :
Let sample mass of AgNO3(s) added be y grams.
=> No of moles of AgNO3(s) added = (y/170) mol
=> [Ag+] added = (y/170)/(250/1000) = 0.023529y mol/dm3
Since molar solubility of Ag2SO4(s) reduced to (0.3121/312.1) = 1x10^-3 mol/dm3Ag2SO4(s) <---> 2Ag+(aq) + SO4 2-(aq)
[ 2(1x10^-3)+ 0.023529y ] [1x10^-3]=> [ 2(1x10^-3)+ 0.023529y ] x [1x10^-3] = 1.4x10^-5
=> (5.5363x10^-4)y^2 + (9.4118x10^-5)y + (-0.013996) = 0
=> y = 4.9437g = 4.94g (3 sf) -
'A' Level H2/H1 Chemistry
Challenging 'O' Level Chemistry
Topic : Redox Calculations5 cm3 of 0.10 mol dm-3 of potassium manganate (VII) reacts with 12.0cm3 of nitrogen monoxide at r.t.p. A colourless solution containing a brown precipitate of manganese (IV) oxide, MnO2 was produced. Determine the final oxidation number of nitrogen in the compound and hence suggest a possible formula of the nitrogen-containing product of the reaction.
Solution :
When MnO4- is reduced to MnO2, only 3e- are transferred per mole of MnO4- used.
5x10^-3 moles of MnO4- implies that 5x10^-4 x 3 moles of electrons are removed from 12/24000 moles of NO.
This implies that 5x10^-4 moles of NO loses 1.5x10^-3 moles of e-.
This implies that each mole of NO loses 3 moles of e-.
Hence, new O.S. = = (+2) + (+3) = +5Therefore, the nitrogen-containing product of the redox reaction could be either dinitrogen pentoxide N2O5, or the uninegative nitrate(V) ion NO3-.
-
Drbiology posted :
Hey guys! Here's a question I can't seem to do. It's in redox topic:
The average oxidation number of the 2 sulfur atoms in sodium thiosulfate, Na2S2O3, is +2.
When solid sulfur containing the radioactive isotope ^35 S is boiled with sodium sulfite, Na2^32SO3, a radioactive sample of sodium thiosulfate is produced. Adding HCl(aq) to this sample causes all the ^35 S to precipitate as sulfur. The resulting solution contains non-radioactive sulfite ions.
i) Draw the structure formula of the sulfite ion.
ii) Use your answer to (i) and the reactions described to suggest a structure for the thiosulfate ion produced. On your structure, label any radioactive atom.
iii) Deduce the oxidation number for each sulfur atom in the thiosulfate ion.
I hope that experts in this forum could help. This assignment is due tmrw btw. thanks in advance! :D
Drbiology, first a warning : the concepts required to properly and fully understand this aspect of chemistry, include formal charges, oxidation states, resonance contributors and resonance hybrids, are almost never properly taught in the JC. Even if you were to ask your JC teacher about these concepts, more often than not, he/she will say, "it's not in the syllabus, you don't need to know, don't ask so much."It's true that it's not strictly required by the syllabus (by which reasoning, this question is an unfair question and therefore you should skip this qn, no?), but without understanding about formal charges, oxidation states, resonance contributors and resonance hybrids, JC students can never have a correct fundamental understanding of chemistry, which is unfortunate.
If you're interested, you can always try asking your JC teacher, or your own private tuition teacher (if you have one), as well as Google and Wikipedia these out yourself (this is afterall, the Age of the Internet, where "All Knowledge is Available to All Mankind").
Solutions :
(i)
Latin name : sulfite ion
Stock name : sulfate(IV) ion
Kekule structure (all 3 resonance contributors as well as the resonance hybrid shown)
http://en.wikipedia.org/wiki/Sulfite(ii)
Latin name / Stock name : thiosulfate ion
Kekule structure (the resonance hybrid shown; the resonance contributors are simply any two of the three O atoms taking turns to be the singly-bonded, negatively formal charged O atoms. Note that Ionic Charge = Sum of Formal Charges.)
http://en.wikipedia.org/wiki/ThiosulfateRadioactive S atom is the terminal (ie. non-central) S atom, of the thiosulfate ion.
(iii) Oxidation Number (ON) = Oxidation State (OS) = Formal Charge + Electronegativity consideration.
OS of the central S atom = (0) + (+4) = +4
OS of the terminal S atom = (0) + (0) = 0
(Notice that for electronegativity consideration, since S and S are 'own people', or as we say in chinese : "zi ji ren" (mandarin) or "kaki lang" (hokkien) or "kaki nang" (teochew) or "ji gei yan" (cantonese), meaning both atoms having the same electronegativity since both are of the same element, hence electronegativity consideration is zero between the two S atoms.)(Technically, there is a slight difference between OS and ON, but for 'A' level purposes they are considered exactly the same thing.)
-
Originally posted by Pinkbow6826:
Iodine and chlorine react together to form compound X (ICln).
When 0.0010 mol of X was reacted with an excess of KI(aq), all of its iodine was converted into I2. The iodine liberated required 40.0 cm3 of 0.10 mol/dm3 sodium thiosulfate, Na2S2O3, for complete reaction.
Calculate the value of n in ICln.
Identify the reactants and products :Reactants : ICln, KI
Products : I2, KCl
Let the coefficient of ICln be 1.
To balance the chlorine, coefficient of KCl must be n.
To balance the potassium, coefficient of KI must be n.
To balance the iodine, coefficient of I2 must be (n+1)/2
Accordingly, write the balanced equation (using coefficients above) for the reaction between ICln and KI to generate I2 and KCl.
By stoichiometry of the balanced redox equation (between iodine and thiosulfate), we note that each mole of ICln generates 2 moles of I2.
By stoichiometry of the balanced reaction between ICln and KI, this implies (n+1)/2 = 2, hence n = 3
-
Organic Chemistry (Synthesis problem)
The common name for the active ingredient in Panadol, is paracetamol. Other names, more descriptive of its structure, are : N-(para-hydroxyphenyl)ethanamide, or para-ethanoylaminophenol.
Q1. Based on the names given above, draw the structure of paracetamol.Ans :
Q2. From the starting material of phenol, propose a synthesis pathway and draw the mechanisms for this pathway, to generate paracetamol.Ans :
Click here for synthesis outline.
From phenol, use dilute nitric(V) acid and dilute sulfuric(VI) acid to carry out mono-nitration. Use fractional distillation to separate the ortho and para isomers.
From para-nitrophenol, reflux with Sn (or Zn or Fe) and excess concentrated HCl(aq), followed by NaOH(aq), to reduce the nitro group to amine. Alternatively, hydrogen with nickel catalyst at 300 deg C may be used for the reduction.
From para-aminophenol, use limited amounts of (ie. as limiting reactant) ethanoic anhydride (or for H2 Chem studens not familiar with acid anhydries, you may use ethanolyl chloride instead) to carry out nucleophilic acyl substitution to generate paracetamol. Notice that the (phenyl) amine group is more nucleophilic than the (phenolic) hydroxy group, because nitrogen is less electronegative than oxygen, and hence more willing to donate dative bonds during nucleophilic attack.
Mechanism wise, H2 Chem students should be able to draw the mechanism for every step of the pathway, except for the reduction of nitro group to amine, and possibly the last step (the mechanism for nucleophilic acyl substitution is addition-elimination, usually taught only to H3 students). But H2 Chem students are encouraged to self-learn this addition-elimination mechanism, because nucleophilic acyl substitution is responsible for organic chem reactions that H2 Chem students should be familiar with, including generating esters, hydrolyzing esters, generating amides, hydrolyzing amides, etc.
-
ElectroChem Qn.
What happens when you mix aqueous tin(II) chloride and acidified aqueous H2O2?
Solution :
There are 5 possible half-equations, 2 reduction half-equations and 3 oxidation half-equations :
H2O2 can be reduced to H2O
Sn2+ can be reduced to SnH2O2 can be oxidized to O2
Sn2+ can be oxidized to Sn4+
Cl- can be oxidized to Cl2Accordingly, there are 6 possible balanced redox equations. Calculate all 6 cell potentials, and select all redox reactions whose cell potentials are positive, and furthermore highlight the redox reaction with the most positive cell potential.
If time is short, and you wish to give only one, most feasible redox equation, here's the shortcut : select the reduction half-equation with the most positive reduction potential, and select the oxidation half-equation with the most positive oxidation potential, add these potentials up to give you the cell potential of the most feasible redox reaction.
-
Topic : Energetics
Originally posted by qdtimes2:1. When 2.76g (0.02mol) of potassium carbonate was added to 30cm^3 of approximately 2moldm^-3 hydrochloric acid, the temperature rose by 5.2drg celcius.
i) write an equation for the reaction
ii) calculate the enthalpy change of this reaction per mole of potassium carbonate. Assume that the specific heat capacities of all solutions have a density of 1.0gcm^-3. Final Answer: -32.6kJmol^-1
iii) Explain why the hydrochloric acid need only be approximately 2moldm^-3.
(i) CO3 2- + 2H+ ---> H2CO3 ---> CO2 + H2O
(ii) Heat transferred = m x c x delta T = 30 x 4.18 x 5.2 = 652.08
Since temperature rose, reaction is exothermic, ie. enthalpy change per 0.02 moles of CO3 2- = -652.08 J
enthalpy change per mole of CO3 2- = -652.08 / 0.02 = -32,604 J = -32.6 kJ/mol
(iii) Based on stochiometry of the limiting reactant CO3 2-, we only need 0.04 mol of H+, implying we only need 1.33 mol/dm3 of HCl(aq). To ensure that CO3 2- is limiting (since our objective is to determine the enthalpy change of reaction per mole of CO3 2-), hence our molarity of HCl should be slightly above 1.33 mol/dm3, for instance 2 mol/dm3.
Added 1 hour later :
Originally posted by qdtimes2:why don't we need to consider the mass of potassium carbonate when calculating the heat involved?
Ans : Because the potassium carbonate isn't the species which absorbed the heat.
Added 2 hours later :
Originally posted by qdtimes2:then why is it that we take into account of the total mass, in let's say a reaction between hydrochloric acid and sodium hydroxide?
Example of the qn in my notes:
50cm^3 of 1moldm^-3 HCl were added to 50cm^3 of 0.65moldm^-3 NaOH in a plastic beaker. The temperature of the resulting solution changed from 21.0 to 25.5 drg celcius. Assuming that it takes 4.2J to increase the temperature of 1cm^3 of solution by 1.0 drg celcius, calculate the enthalpy change of neutralisation per mol of water.Answer: Q = (50+50) x 4.2 x (25.5 - 21.0) = 1.89 kJ
Ans : Because the reactants in this particular question are aqueous, meaning the water from these aqueous reactants must be considered to have absorbed the heat evolved.
-
ACJC 2008 Prelims P3 Qn :
Why does chlorine turns moist starch-iodide paper blue?
Answer :
The reason is that chlorine has the capacity to oxidize (by accepting electrons) iodide to iodine, and certain polyatomic iodine complexes are able to, by virtue of 3-dimensional helical configuration compatibility, electromagnetically interact (at the electron orbital levels) with certain polymers of amylose starch to (have their inter-orbital electron transitions) absorb primarily the wavelengths of the electromagnetic spectrum corresponding to the complementary colour of that which is observed, resulting in the human eye perceiving, and the human brain interpreting, the colour of the starch-iodine complex as dark blue in colour.
-
Cyyn asked :
Chem SPA Qn : What's the main source of error for the displacement method to find the volume of gas produced when acid reacts with a carbonate? the experiment is like, a burette with a tubing connected to the conical flask and another tubing connected to a funnel and water is filled into the burette through the funnel.. (the tubing from the conical flask doesn't touch the water) And 'Compare the reliability' between that method and an experiment with the tubing connected to the bottom of the burette (into the water).
Answer :
Hydration of gaseous CO2 (to a small extent) and hydrolysis of gaseous/aqueous CO2 (to a larger extent) will result in a lower-than-expected volume of gaseous CO2 collected.
Hydration : CO2(g) + aq ---> CO2(aq)
Hydrolysis : CO2(g/aq) + H2O(l) ---> H2CO3(aq) ---> H+(aq) + HCO3-(aq) -
Compound R (formula unknown) has a molar mass of 289g (to 3 sf), and exists as stereoisomers R1 and R2. When refluxed with hot alkaline KMnO4 followed by acidification, R gave two products : S1, a compound that after reacting with LiAlH4 (in dry ether) followed by protonation via hydrolysis, became converted to a primary alcohol that generated a yellow precipitate with alkaline aqueous iodine; and S2, C4H6O3.
S2 generated yellow crystals with alkaline aqueous iodine solution. S2 also generated a non-polar, acidic gas when mixed with a white powder (of molar mass 84g).
R when heated with hot ethanolic KCN generated T,C8H10N2. T reacted with H2 and nickel catalyst at 140 deg C to generate U, C8H20N2. With aqueous hypobromous acid (latin name) or bromic(I) acid (stock name), U generated an white crystalline solid, V1, C8H22N2Br2O2, when the aqueous mixture was dehydrated at low temperatures. When V1 was warmed to room temperature, V1 disproportionated into crystalline solids V2, C8H22N2Br2 and V3, C8H22N2Br2O6 (in the molar ratio of 2:1 respectively).
T when heated under reflux with a dilute acid generated W, C8H12O4, which reacted with a heated mixture of chlorine and phosphorus (either white P4 or red P) to generate X, C8H10O2Cl2, one mole of which reacted with two moles of an alkaline gas (molar mass of 31g) to generate Y, C10H18O2N2, a non-basic, nitrogen-containing compound (non-basic because the lone pair on the N atom is 'locked up' by resonance to give partial double bond character with the adjacent C atom, and is hence unavailable to accept a proton).
Elucidate R1, R2, S1, S2, T, U, V1, V2, V3, W, X, Y.
(Note : there are 2 possible structural isomers for R; for either structural isomer, draw the two stereoisomers R1 and R2.)Answers :
R is CH3(X)C=C(CH3)CH2CH2X where the two X atoms are I and Br (ie. one of each), hence 2 structural isomers are possible and either accepted. R1 and R2 refer to the two geometric isomers possible.
S1 is CH3COOH
S2 is CH3COCH2COOH
T is NC(CH3)C=C(CH3)CH2CH2CN
U is H2NH2C(CH3)CHCH(CH3)CH2CH2CH2NH2
V1 is BrO-+H3NH2C(CH3)CHCH(CH3)CH2CH2CH2NH3+-OBr
V2 is Br-+H3NH2C(CH3)CHCH(CH3)CH2CH2CH2NH3+-Br
V3 is BrO3-+H3NH2C(CH3)CHCH(CH3)CH2CH2CH2NH3+-O3Br
W is HOOC(CH3)C=C(CH3)CH2CH2COOH
X is ClOC(CH3)C=C(CH3)CH2CH2COCl
Y is H3CNHCO(CH3)C=C(CH3)CH2CH2CONHCH3Note : While geometric isomerism exists for R, T, W, X and Y, you are required to draw the two geometric isomers for compound R only (ie. as R1 and R2).
-
^_^
-
H2 Chemistry Qns.
1) Why 4-nitrophenylamine is less basic than 4-chlorophenylamine?
2) Why is phenylethanoic acid is a weaker acid compared to benzoic acid?
3) Which of the following will not be formed when C6H5CH2CH2CI reacts with hot ethanolic KOH
A) C6H5CH=CH2
B)C6H5CH2CH2OCH2CH3
C)C6H5COOH
D) C6H5CH2CH2OH4) In oil refineries, an important process is the recovery of any sulphur from
petroleum.
2H2S(g) + O2(g) � 2H2O(g) + 2S(s)
The enthalpy change of formation of H2S(g) is -20.5 kJ mol�1 and that of H2O(g)
is -243.0 kJ mol�1.
Which statements are true?
1 The above reaction is thermodynamically feasible only at low
temperatures.
2 Enthalpy change of combustion of H2S is -445 kJ mol�
3 Enthalpy change of above reaction can be calculated from bond energy
data of reactants and products.Hints :
Q1. The nitro group withdraws by induction and resonance both, while chlorine only withdraws by induction (and donates by resonance). The more electron withdrawing the substituent, the less available the lone pair on the N atom to accept a proton.
Q2. Phenylethanoic acid is a weaker acid compared to benzoic acid, because phenylethanoic acid has an additional C atom to donate electrons inductively to the COO- group of the conjugate base, which has a destabilizing effect (due to increased inter-electron repulsion).
Q3. Heating under reflux with KMnO4 is required to accomplish C.
Q4.
You're Emperor Gribbs. Of your two prime ministers, Mr Entropy (prime minister) doesn't support you (entropy change is negative), while Mr Enthalpy does (enthalpy change is exothermic). Therefore, you must punish Mr Entropy by making his wife, Temperature, cold-cold (so she can't pleasure her husband).
The extraction reaction given isn't combustion (it only looks like it, because O2 is a reactant). For the combustion enthalpy of H2S, you'll need the formation enthalpies of H2O and SO2 or SO3, depending on which combustion equation is used.
Bond enthalpies given in the Data booklet only hold true for gaseous bonds. -
^_^
-
H2 Chem Qns.
Originally posted by gohby:Hey guys, I've yet another couple of chem questions and I'm hoping that any chem pros here can help me out..
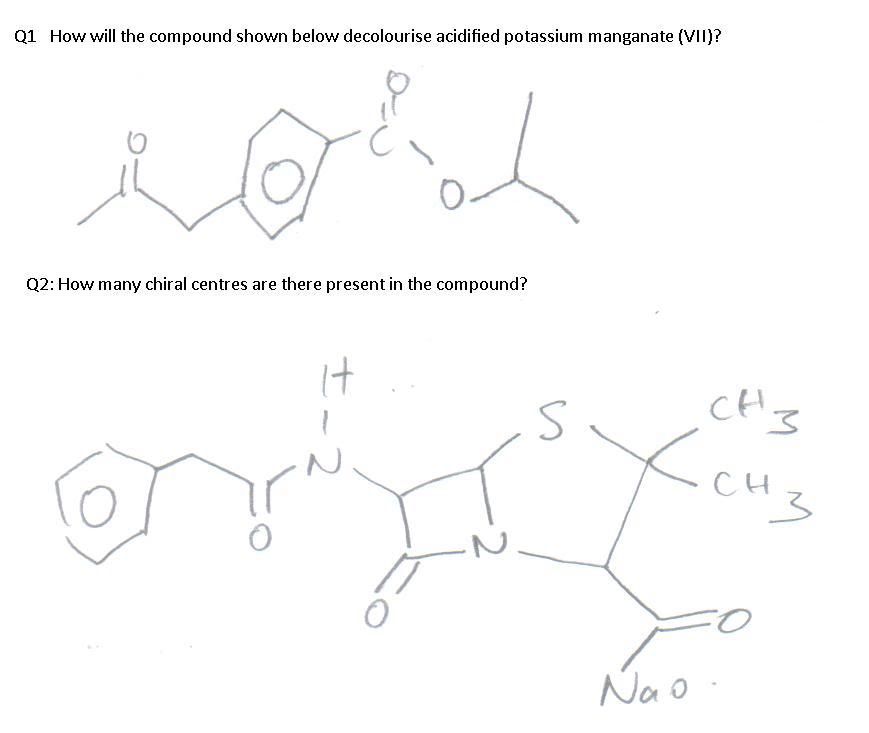
For Q1, I don't see how KMnO4 is going to be decolourised but the answer suggests as such so I want to verify if I've made an oversight.
For Q2, I have counted 3 chiral centres, but the answer suggests 2. (Btw, do I consider counting chiral nitrogens into account)?
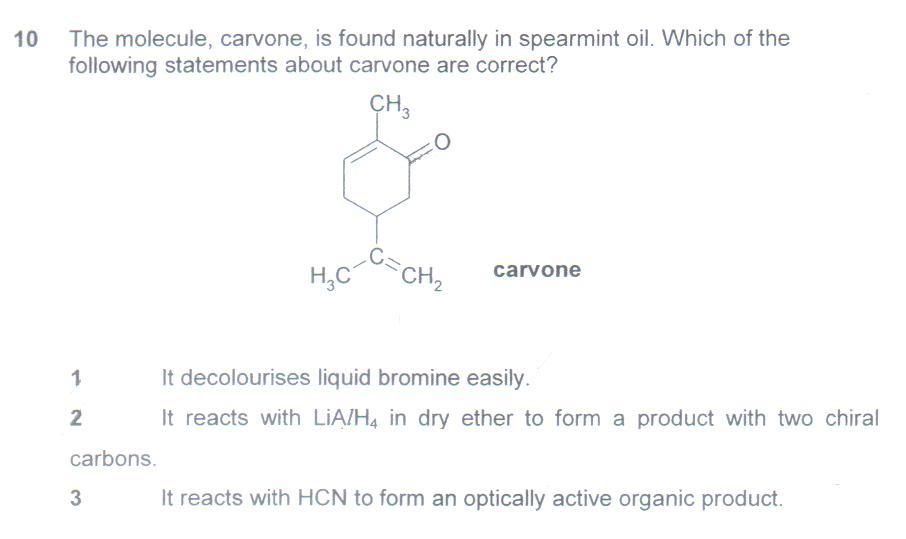
The answer is 1 & 2 but I worked out the answer as 1 & 3 instead.

Q23 - A modified question from N07 A levels. Firstly, what exactly constitutes a CFC? Apprently A is a CFC even though it doesn't contain chlorine or fluorine.. And D is the answer even though it contains fluorine! :S
Another N07 question. Simple question but forgot how to do.. :X
Thank you very much in advance! :D Much appreciated! :)
Q1. The alkyl side chain of the benzene ring on the left will be oxidized, generating a benzoic acid functional group. When heated under reflux with KMnO4 (the requirement to oxidize alkyl side chains of benzene rings in any case), the other side chain will undergo hydrolysis, and subsequently (the products of hydrolysis) oxidation as well.
Q2. Yes there are 3 chiral carbons, 2 in the lactam ring, 1 outside. For H2 Chem, Cambridge will only ask you to count chiral carbons, not chiral nitrogens. There is another difficulty associated with chiral nitrogens, namely nitrogen inversion. See : http://www.rod.beavon.clara.net/chiralit.htm and http://en.wikipedia.org/wiki/Nitrogen_inversion
Q3. 1 and 2 is correct. Option 3 is wrong because the reactant is trigonal planar, and thus the nucleophile can attack from either above or below, and therefore the product is generated as an optically inactive racemic mixture, even though chiral carbons are present. Option 2 is correct because of a new chiral carbon being the alpha carbon of the primary alcohol generated, as well as the preexisting chiral carbon at the base of the hexane ring. Note that LiAlH4 does not reduce alkenes, because both (H- from LiAlH4 and alkenes) are nucleophilic and thus repel each other (ie. they're not gay / homosexual. Nucleophiles are guys who only like electrophiles whom are girls. Only nucleophiles have the 'balls' to attack electrophiles, the pair of 'balls' referring to a lone pair usually, although for benzene nucleophile and alkene nucleophiles a pi bond is used. The 'balls'/lone-pair shoot out to become a bond-pair with the electrophiles/girls, who happily accept them, since they are 'balls'/electron-deficient)
Q4. D is indeed the answer. Don't focus on the 'definition of a CFC'. Focus instead on using a compound in which there are no C-Br or C-Cl bonds, and only C-C, C-H and C-F bonds. C-F bonds do not cleave readily (short bond length = strong bond), and therefore pose much less of a threat to the ozone layer (depleted by free radical substitution reactions), unlike compounds with C-Cl bonds or C-Br bonds which are homolytically cleaved by UV radiation in the stratosphere, generating Cl. or Br. radicals which attack ozone.
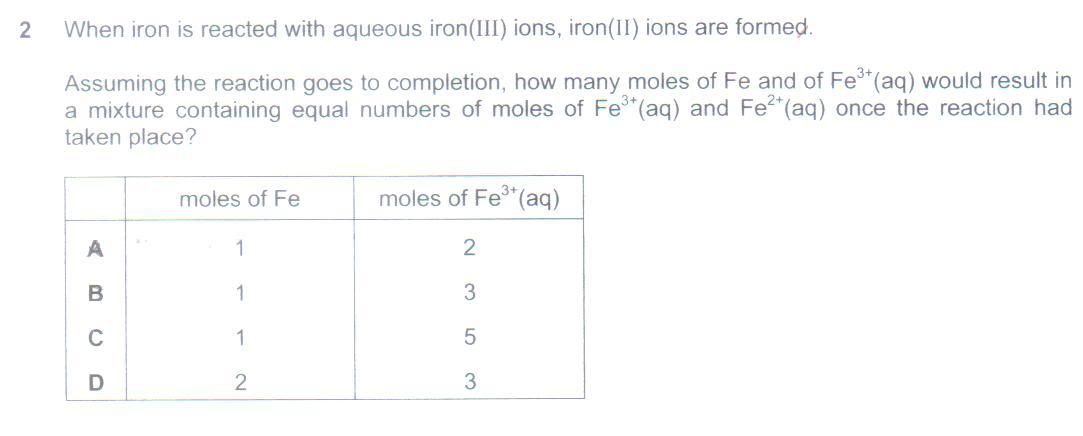
Q5. Do an ICF (Initial Change Final), not ICE (Initial Change Equilibrium) table. Don't use ICE because there isn't any equilibrium to speak of here. Write the balanced redox eqn. Do the ICF table. Since common (chemistry) sense will indicate that Fe is limiting and Fe3+ is in excess, Let the Initial moles of Fe be 1, and Fe3+ be X (remember, your best friend in Chemistry calculations is.... Algebra!), and Fe2+ be 0. Then Change will be -1, -2 and +3 (based on stoichiometry of the balanced redox eqn). Therefore Final will be 0, X-2 and 3. Since at Final, there are equal moles of Fe2+ and Fe3+, hence X-2 = 3, and therefore X = 5. Accordingly, the required mole ratio is 1 Fe : 5 Fe3+.
-
ilovee37e posted
Hello, Thanks Ultima for the previous reply! I have doubts once again.
1) Why Beryllium chloride can conduct electricity in the solid state?
2)The bond lengths in buta–1,3–diene differ from those which might be expected.
The carbon – carbon bond length in ethane is 0.154 nm and in ethene 0.134 nm. The central single bond in buta–1,3–diene (C2 – C3), however , is shorter than the single bond in ethane: it is 0.147 nm.
1 2 3 4
CH2 CH CH CH2
0.134 nm 0.147 nm 0.134 nm
What helps to explain this C2–C3 bond length?
A)It is an sp2 – sp2 overlap.
It is an sp2 – sp3 overlap.
The electrons in the filled p orbitals on C2 and C3 repel each other.
The sp3 – sp3 bonding is pulled shorter by p–p ( π – bond ) overlap.
Why is the C2-C3 longer than that in ethene? Is it because C2-C3 is connected by a single bond,thus it is longer while assuming that C2-C3 are sp2-sp2 overlap in the same scenario in ethene?
3)Which of the following statements about a solution equimolar with respect to both benzoic acid and sodium benzoate is incorrect?
A It has a pH value equal to the pKa value of benzoic acid.
B Its pH value remains constant when diluted with water.
C It can be prepared by mixing 50 cm3 of 0.1 mol dm-3 hydrochloric acid with 50 cm3 of 0.2 mol dm-3 sodium benzoate.
D It can be prepared by mixing 50 cm3 of 0.1 mol dm-3 benzoic acid with with 50 cm3 of 0.2 mol dm-3 sodium hydroxide.
The answer is D but i do not understand why it is so.
4)State the formula of the oxide of titanium that can be used as a white pigment in paint.
A TiO B Ti2O3 C TiO2 D TiO3
Hmm,no idea for this question -_- no concepts came to my mind.No prob, ilovee37e.
Q1. It can't. BeCl2 exists as a polymeric covalent lattice in the solid state, and (predominantly) as simple covalent molecules in the liquid (molten) and gaseous states (due to the high charge density on the small Be2+ ion). Accordingly, it does not conduct electricity (well) in any state.
Q2. The sp2-sp2 sigma bond (in buta-1,3-diene) is shorter than the sp3-sp3 sigma bond (in ethane) because the sp2-sp2 sigma bond has greater % s character (since s orbitals are closer to the nucleus than p orbitals).
Q3. Do the ICF (Initial Change Final) table, if you can't work it out mentally. Option D results in equal molarities of OH- and C6H5O-. There is no C6H5OH left, and thus cannot function as a buffer solution.benzoic acid + hydroxide --> benzoate
Initial | 5x10^-3 | 0.01 | 0
Change | -5x10^-3 | -5x10^-3 | +5x10^-3
Final | 0 | 5x10^-3 | 5x10^-3From the ICE table, we note that at the end (Final) of reaction, we have equal moles, and hence equal molarities, for hydroxide ions and benzoate ions. This is NOT a buffer solution (because hydroxide ions and benzoate ions are both basic). To generate a buffer solution, we require similar (and at maximum buffer capacity, EQUAL) molarities of the weak acid (in this case, benzoic acid), and it's conjugate base (in this case, benzoate ions).
Q4. Titanium is a transition metal. Based on its d-orbitals, which of its ions are coloured, and which are non-coloured (ie. white)? -
H2 Chem + H2 Bio
Cyyn asked :
Assuming that only the 20 commonest amino acids, are present in a given organism, how many different dipeptides might theoretically be found in it?
UltimaOnline replied :If you have 20 chiral carbons, with each chiral carbon having 2 possible configurations : dextrorotatory(+) or levorotatory(-), then you can have a maximum (assuming no internal planes of symmetry) of 2^20 = 1,048,576 possible optical isomers.
If you have 2 amino acid residues (ie. a dipeptide), with each amino acid residue having 20 possible identities, then you can have a maximum of 20^2 = 400 possible dipeptides. -
'O' levels + 'A' levels.
Originally posted by Blasst:1)The mass of one mole of a chloride formed by a metal Y is 74.5g.The formula of the chloride could be
A)YCl
B)YCl2
C)Y2Cl
D)Y2Cl2
2)Aluminium is higher up in the reactivity series than iron.however,iron rusts (reacts with air and water) buy aluminium does not.What is the reason for this?
A) An inert oxide coating formed on aluminium.
B)aluminium oxide is amphoteric.
C)Iron (II) oxide is water soluble.
D)Iron (III) oxide is a basic oxide.
3)Which metal has the least tendency to form positive ions?
A)Aluminium
B)sodium
C)calcium
D) copper
4)a piece of magnesium ribbon does not react when put into a solution of hydrogen chloride in methylbenzene,an organic solvent. Which change will cause this reaction to occur?
A)adding water and stirring.
B) ADding more methylbenzene.
C)stirring the mixture vigorously.
D)boiling the mixture.
5)In which compound is nitrogen in its lowest oxidation state?
A)N2O
B)NO
C)NO2
D)N2O2
These are all 'O' Level Pure chemistry mcq questions :)
Q1.
This is a not a usual type of chem calculation question, since trial-and-error is required. However, because the molar mass of 74.5g isn't too large, the answer can be obtained by the average student within 30 seconds. The chloride is potassium chloride, KCl, molar mass 74.6g.
Another example of a similar question : which hydrocarbon has a molar mass of 26g? Ans : ethyne.
Q2.
This inert layer of metal oxide, is known as the "passivation layer", since it makes the metal relatively unreactive. (It's not that the metal oxide is totally unreactive, but just less reactive than the pure metal itself).
Q3.
The more reactive the metal, the more positive it's oxidation potential, the greater the tendency to form cations. Copper is the least reactive (standard oxidation potential of Cu to Cu2+ = -0.34V). Based on the standard redox potentials given in the Data Booklet, (note : reduction potentials are given, oxidation potentials can be deduced by changing the +ve/-ve sign), can you deduce which is the most reactive metal among Al, Na and Ca?
Oxidation potentials of Al, Na and Ca are +1.66V, +2.71V and +2.87V. Accordingly, the most reactive metal (ie. the metal with the most positive oxidation potential) is Ca.
Q4.
'A' level answer :Acidic gases such as hydrogen halides (eg. hydrogen chloride) do not behave as (Bronsted-Lowry) acids, meaning they do not dissociate or donate H+ ions, when dissolved in non-polar, organic solvents such as methyl benzene. They remain as covalently bonded H-Cl molecules in such non-polar organic solvents. In contrast, with polar solvents (especially protic polar solvents) such as water, H-Cl molecules ionize (ie. form ions) by dissociating into H+ ions and Cl- ions. The H+ ions are actually present in the form of H3O+ ions (hydroxonium ions or hydronium ions). The reason for this different behaviour of hydrogen halides in non-polar solvents versus protic polar solvents, has to do primarily with enthalpy considerations (compare magnitudes of the exothermic ion-dipole interactions with the endothermic bond dissociation process) and to a smaller extent, entropy considerations.
'O' level answer :
Acids only behave as acids when dissolved in water.
Q5.
The 'O' level method for finding OS (Oxidation State), involves recognizing that "Ionic charge is the sum of atomic OSes". Let the OS of N be x. OS of each O atom is -2 (unless O is bonded to another O atom, or to more electronegative F atom, both of which is rarely asked at 'O' levels). Solve for x.
Eg. N2O2, we have 2x + 2(-2) = 0, hence x = +2
A)N2O has N with OS of +1
B)NO has N with OS of +2
C)NO2 has N with OS of +4
D)N2O2 has N with OS of +2
The BedokFunland JC formula is OS = Formal Charge + Electronegativity consideration.
Eg. N2O2, there are several resonance contributors we can use, most notably the following two :
Contributor #1
O atom doubly bonded to N atom singly bonded to N atom doubly bonded to O atom. For each of the N atoms, OS = Formal charge + Electronegativity consideration = (0) + (+2) = +2
Contributor #2
-ve O atom singly bonded to +ve N atom triply bonded to +ve N atom singly bonded to -ve O atom. For each of the N atoms, OS = Formal charge + Electronegativity consideration = (+1) + (+1) = +2
-
^_^
-
Blacktoast94 asked :
>>> explain why aluminium chloride is al2cl6 at 200 degrees but becomes alcl3 at 800 degrees. <<<
UltimaOnline replied :
You can think of it in 3 ways : equilibria, activation energy to break bonds, and energy levels of AlCl3 vs Al2Cl6. When you see the big picture of chemistry, you'll appreciate that all 3 ways of looking at it are really all different aspects of viewing the same phenomenon.
Yes, AlCl3 exists in equilibrium with Al2Cl6, because both are comparably stable in different ways (stable octet versus formal charge).
Yes, converting Al2Cl6 to AlCl3 is endothermic, since you're breaking the (dative) covalent bonds.
Yes, smaller AlCl3 molecules have higher kinetic energy, compared to bigger and slower Al2Cl6 molecules.
Therefore, heating will result in providing activation energy to break the (dative) covalent bonds to monomerize Al2Cl6 back to AlCl3, and shifts the position of equilibrium over to the side of AlCl3, since in a higher energy (ie. hot!) environment the species naturally also has higher energy, and aluminium chloride feels more comfortable to exist as the higher energy AlCl3 rather than the lower energy Al2Cl6.
Just like when you become richer, you're feel more comfortable wearing more fashionable clothing or eating at higher quality restaurants, rather than wearing old and worn clothes, or eating from lower standards hawker center or food court.
(Disclaimer : the above is just a didactic example used for pedagogical purposes; I personally appreciate humble clothing and good hawker food.)
------------------------
Blacktoast94 asked :could u also explain to me why ionic cpds cannot dissolve in organic cpds? i know why but dunno how to put it in words.
Newcharacter offered :No ion dipole bond is formed to cause the release of energy for the breaking down of lattice structure for solvation of ionic compounds in organic compound.
UltimaOnline finished off with a headshot :Correct. In contrast, with protic polar solvents such as water, (relatively) stronger ion-dipole interactions (ie. ion-dipole "bonds" formed between the ions and the strongly polar water solvent molecules) result in sufficient heat energy released to compensate for the endothernic lattice dissociation process (ie. breaking of ionic bonds), hence some ionic compounds are soluble in water.
(Notice that just as there is permanent dipole - permanent dipole van der Waals, versus permanent dipole - induced dipole van der Waals, versus instantaneous dipole - induced dipole van der Waals, similarly there is ion - permanent dipole (for polar solvent, eg. water) and ion - induced dipole (for non-polar solvent). Ion - permanent dipole is naturally significantly stronger than ion - induced dipole (which explains why some ionic compounds are soluble in water but not in non-polar solvents), but for 'A' level purposes, you can just use the term "ion-dipole" to imply "ion - permanent dipole" interactions.)
Notice that when the charge density on the cation is relatively low (eg. Na+), ion-dipole interactions result. The strength of these ion-dipole interactions increase with the charge density of the cation, but only up to a certain point. When the charge density on the cation becomes too high (eg. Al3+), the nature of the solute-solvent interactions shift from ion-dipole interactions, to coordinate dative covalent bonds donated from the water molecules which accordingly behave as ligands. This involves a subtle transition, and something JC students needn't worry too much about.
There is also the matter of entropy to consider. Because many soluble ionic compounds, eg. ammonium salts, even after considering that solution enthalpy = endothermic lattice dissociation enthalpy (ie. breaking ionic bonds) + exothermic hydration enthalpy (ie. forming ion-dipole bonds), will be noted to have endothermic solution enthalpies. So based on enthalpy alone, you cannot explain why ammonium salts are soluble. For such cases (eg. ammonium salts), you ultimately have to bring in entropy, in the Gibbs free energy formula, to explain why these ionic compounds are soluble.
-----------------------------------
Blacktoast94 wrote :Thanks for the detail, as always.
UltimaOnline added :You're welcome, as always. Btw, one more thing :
Notice that because Be, Al and Ge have a 'diagonal relationship' in the periodic table, their cations' charge densities are similar and hence their oxides/chlorides may be expected to have similar (to some extent) properties. Eg. the oxides of these 3 are somewhat amphoteric, and the chlorides of these 3 have significant covalent character.
Furthermore, you should already be aware (since 'O' levels) that the oxide of Pb is amphoteric, and since the oxide of Po may also expected to be somewhat amphoteric (since Po has diagonal relationship with Al), therefore you can deduce that it is likely that the oxide of Bi (which is directly between Pb and Po) may also be somewhat amphoteric.
This 'diagonal relationship of elements' is only a limited guide of course, for in reality chemistry is a lot more complex (there are always mutliple factors involved, and some of these are often opposing) than the Periodic Table can possibly cover (unless of course you have the Ultimate Periodic Table). -
^_^
-
Geneva asked :
what are the biological processes that produce free radicals?
UltimaOnline replied :
Tis the Age of the Internet, where All Knowledge is Freely Available to All Mankind (well not quite all knowledge, the United States government will not yet publicly admit that they have been well aware of extraterrestrial presence on this planet for decades now, for several important reasons (some of them selfish, such as the technological aspect), including the very real concern of causing worldwide socio-political, religious, economic and military instability).
Google by thy Sword, Wikipedia be thy Shield.
http://en.wikipedia.org/wiki/Radical_(chemistry)
(The above Wikipedia entry includes the biological perspective you seek.) -
ilovee37e asked :
> When a sample containing 2 mols of SO2Cl2 is placed in a 2dm3 vessel at 30 degree celcius, it decomposes to So2 and CI2. At eqm, the total pressure of mixture is determined to be 38.9atm. Find the molarities of each gas at eqm. <
Solution :
P V = n R T
(38.9)(2) = n (0.0821) (273 + 30)
n = 3.127 moles
SO2Cl2 ---> SO2 + CI2
(moles) 2 | 0 | 0
(moles) -x | +x | +x
(moles) 2-x | x | x
Since total moles of gas at equilibrium = 3.127 moles, this implies :
2-x + x + x = 3.127 x = 1.127 moles.
At equilibrium,
moles of SO2Cl2 = 0.873 mol, hence molarity of SO2Cl2 = 0.4365 mol/dm3
moles of SO2 = 1.127 mol, hence molarity of SO2 = 0.5635 mol/dm3
moles of Cl2 = 1.127 mol, hence molarity of Cl2 = 0.5635 mol/dm3 -
'A' level / 'O' level
Stoichiometry Qn
ilovee37e posted :
Pyrogallol is a white crystalline powder and a powerful reducing agent that is used to absorb O2. When 30cm3 of a gaseous hydrocarbon is exploded with 200cm3 of O2, there is a contraction of 90cm3. On furthur treatment with alkaline pyrogallol, there was a reduction of 20cm3.
And the question is : Who will win the Aljunied GRC?Digressing from this all-important question, let's have a look at the pyrogallol discussion (there doesn't seem to be a question there though).
The 20cm3 reduction shows that excess O2 = 20cm3. Which means O2 used up = 200-20 = 180cm3.This implies 30cm3 of hydrocarbon used up 180cm3 of O2.
So 30(X + Y/4) = 180
That's one equation.Overall contraction of 90cm3, implies initial volume of gas - final volume of gas = 90cm3
So (230) - (30X + 20) = 90
30X + 20 = 140
X = 4Sub into earlier equation, 30 (4 + y/4) = 180
(4 + y/4) = 6
y/4 = 2
y = 8Therefore, the hydrocarbon is C4H8, which is either but-1-ene, but-2-ene, cyclobutane, or methylcyclopropane.
-
hmyatthazin asked :
I'm having problems balancing this redox equation. The reaction is between Mn2+ and O2 producing Mn(OH)3. I tried using half equation method but it doesn't work. Must I use oxidation number method? I also tried but couldn't get the answer. I'd really appreciate if you can help me :) Thank you :)
UltimaOnline rescued with :
The trick to writing all redox equations, is to identify (based on the oxidation state, the OS) exactly what is being oxidized, what is being reduced, and to write separate half-equations for each first, before combining them to get the balanced redox (ie. overall) equation.[Reduction] OS change of oxygen is from 0 to -2
O2 ---> OH-
O2 ---> 2OH-
2H+ + O2 ---> 2OH-
4e- + 2H+ + O2 ---> 2OH-[Oxidation] OS change of manganese is from +2 to +3
Mn2+ ---> Mn3+
Mn2+ ---> Mn3+ + e-
4Mn2+ ---> 4Mn3+ + 4e-[Balanced Redox]
4Mn2+ + 2H+ + O2 ---> 4Mn3+ + 2OH-
10OH- + 4Mn2+ + 2H+ + O2 ---> 10OH- + 4Mn3+ + 2OH-
2H2O + 8OH- + 4Mn2+ + O2 ---> 12OH- + 4Mn3+
2H2O + 8OH- + 4Mn2+ + O2 ---> 4Mn(OH)3The last part is indeed tricky, because Mn(OH)3 exists as a solid precipitate and must be written as such (ie. not as separate aqueous ions). Therefore, we add 10 OH- ions on both sides (since we need 12OH- on the RHS), thus obtaining 2H2O + 8OH- on the LHS, and finally on the RHS we combine the 12OH- + 4Mn3+ to obtain the required 4Mn(OH)3 solid precipitate.
-
Another qn: even though xef4 has more electrons and is hence expected to have greater intermolecular forces than xef2, it's melting pt is lower. Give a reason why. Help is appreciated thanks
UltimaOnline posted :
This is an example of a question that 'A' level students will not be expected to be able to correctly answer.
The melting point of a compound, depends on how well they fit into a solid lattice structure (this is the reason why the cis isomer of alkenes have a higher boiling point, but a lower melting point, compared to the trans isomer).
Because the molecular geometry of XeF4 is square planar, with the central Xe atom partially positively charged, and all 4 F atoms in a square planar arrangement partially negatively charged, it becomes difficult to pack these molecules closely in a solid lattice, without incurring significant van der Waals repulsion between the partially negatively charged F atoms.
In contrast, the XeF2 molecules, although non-polar, are able to be stabilized in close proximity (as a lattice structure in the solid state) with permanent dipole - permanent dipole attractions, in a similar way that non-polar CO2 molecules can be stabilized by permanent dipole - permanent dipole attractions in the solid state as dry ice (though for 'A' levels, Cambridge won't expect students to be able to understand this, and will accept the simpler model of instantaneous dipole - induced dipole van der Waals like all other non-polar molecules).
(Additional note : Having said that, there is a significant difference between CO2 and XeF2, which is : in CO2, the central partially positively charged C atom has no lone pairs, while the central partially positively charged Xe atom has 3 lone pairs. Therefore the (relative) magnitude of permanent dipole - permanent dipole attractions will be expected to be more significant for CO2 than for XeF2. And yet XeF2 has a much higher melting point than CO2, because XeF2 has a lot more electrons, has much larger molecular size, and therefore has significantly more polarizable electron charge clouds.)
However, all the above is still just the tip of the iceberg, and there are multiple other underlying reasons, often competing and/or contradictory, that determines the final melting points of XeF2, XeF4 and CO2. As mentioned at the beginning of this post, 'A' level students are not expected to be able to fully understand all of this.
It is worth noting, that XeF2 can melt into the liquid state (ie. continues to experience significant van der Waals attraction between molecules as a liquid), while XeF4 sublimates, implying that van der Waals repulsion between the partially negatively charged, square-planar arranged F atoms of the poorly lattice-fitting molecules, as described in preceding paragraphs outweighs the van der Waals attraction resulting in the lack of a liquid state, at least under standard pressure conditions.
It is further worth noting, that at higher and highest pressures, experimental evidence have shown that XeF2 is able to solidify into semi-metallic (graphite-like) and fully metallic allotropic structures, respectively. This is not possible with XeF4, for reasons described above.
Finally, if you've understood at least most of this discussion, you should be able to extrapolate : based on the molecular geometry of XeF6 versus XeF4 and XeF2, how do you expect the melting point of XeF6 to compare with that of XeF4 and XeF2?
Answer :
the melting point of XeF6 is even lower than XeF4 and XeF2, due to the more significant van der Waals repulsion (or less significant van der Waals attraction) between the partially negatively charged F atoms arranged in an octahedral geometry about the central, 'protected', partially positively charged Xe atom.