Hi gohby, you're totally welcome. 
By the way, out of curiosity, are you a secondary school student yourself, a concerned parent of a secondary school child, or a secondary school tuition teacher (if so, are you a full time professional tutor, or are you a tertiary student giving tuition on the side, or a working adult giving tuition on the side)?
Regarding Qn1 :
For the O level syllabus, they should be familiar with the QA test for nitrate(V) ions, which does include aluminium metal and aqueous sodium hydroxide. Yes, it may be considered one of the more challenging or discriminating questions at 'O' levels, but it is nonetheless still a fairly reasonable one that could be asked at in the actual 'O' levels, for both combined science Chem and pure Chem.
So even if they have no idea on why it works (ie. my explanation in my previous post), but they could still correctly answer the question by recall of the QA test for nitrate(V) ions.
There is a separate point to be made here. You hear the saying how everywhere around the world, in China, in Korea, in Japan, in the UK, etc, and certainly in Singapore, more and more people are getting University degrees, such that soon people would need a degree to 'sweep roads'. This is a actually a sad state of human affairs, and isn't just a localized Singaporean problem. (Though having hundreds of thousands of foreigners coming in every year competiting with locals, students or working adults, while (the foreigners themselves are only fellow humans striving for a better life) not the root of the problem, does exacerbate and accelerate the pressure-cooker societial problems faced by children students in the Singapore education system.
All parents want the best for their children. And all children are getting increasingly desperate to be at the 'top'. You hear sadly ridiculous stories about how almost every parent whose child is not above average in academia, gets all upset with the child, "why are you not above average? Being average means you're a failure in society!". This attitude is hypocritical and unethical, because either it means such parents have no idea what the mathematical definition of "average" is, or (far more likely) it means such parents look down upon everyone else.
It is the same within the MOE system. Principals (for the sake of their own promotion) put pressure on HODs, who (for the sake of their own promotion) in turn put pressure on teachers. "Our school results *must* be better than the national average! If not, something is *wrong*, and heads will roll!" So if everything is 'right' as it 'should be', then *everyone* would be scoring 'above average', which is a mathematical, logical impossible fallacy. Or more to the point, reeks of elistist, anti-ethical, anti-compassionate, separatist, ruinous attitudes.
This dog-eat-dog survival-of-the-fittest unethical behaviour, is to the detriment of mankind. Humanity risks destroying itself, if technological advancement is not accompanied by compassion, empathy, ethical and consciential evolution.
So every year, the number of students seeking to enter University increases; every year the number of students working and studying harder and desperately, increases; every year the number of students who obtain good results and apply into University increases; but yet the number of available University courses are not commensurate with demand. (And not forgetting competition from increasing number of foreign students for limited places in local University courses. The foreigners are not the problem, the system is. The world financial system is unethical and flawed. Humanity is heading towards trouble.).
Every year we have many thousands of medical doctors from China, India, etc being imported into Singapore, because the government says "We don't have enough doctors". And yet every year many thousands of local JC & Poly students who score all perfects A's, apply for Medical school but are rejected, due to the very small number of places available; the families of these students then spend hundreds of thousands of dollars to send them overseas for Medical school, incurring not just financial debt but emotional bitterness.
Regarding Qn 3 :
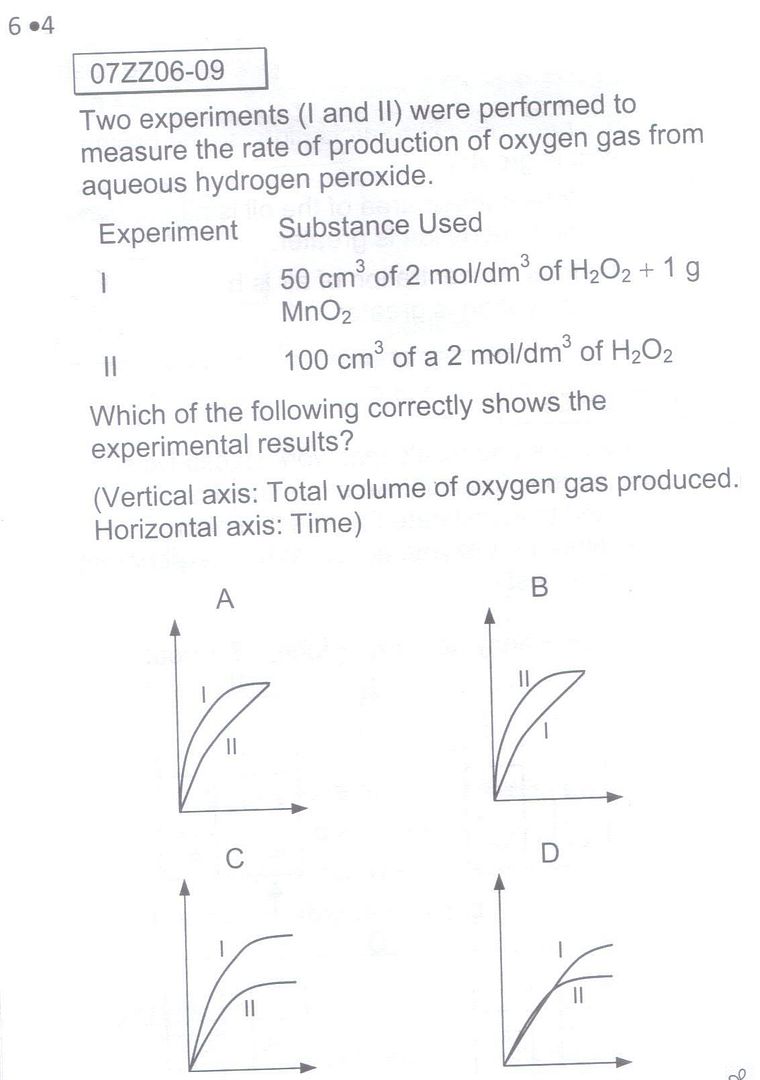
Exp I would indeed be faster (a lot faster actually, the shape of the graphs are grossly inaccurate) due to the presence of a catalyst, the manganese dioxide. As to the truncation, it is arguable as to whether it is a fair question or not. I would be inclined to agree with you on this, but the fact is exams (both internal and national level) are increasingly relying on such 'despicably' tricky questions, in a bid to decrease the number of A grades, to steepen the bell-curve, to make examinations tougher year-on-year.
Because more and more parents and students are getting desperate with the increasing costs of living and increasing competition from foreigners, so more and more students are working harder and getting better grades. And more and more people are getting University degrees (something like 80% of Uni grads in China/Korea/etc are unemployed).
The exam setters are getting alarmed at how more and more students are doing well and getting As. So they feel pressured that they've to set tougher and tougher questions; just as year-on-year, every next generation of students feel increasingly pressured that they've to do better and better than everyone else, to be 'better than average', for the less-and-less (relative to population increase) available places in the University, for less-and-less decent paying jobs (because the rich keep getting richer and the poor keep getting poorer, the top-down pyramid in the corporate world keeps getting more and more elitist at top, more and more poor masses at the bottom; less than 10% of the richest individuals own more than 90% of the planet, etc. The richer you are, the more opportunities you have to increase your wealth. The poorer you are, the less opportunities you have to increase your wealth. This isn't about Singapore. It's about the divide between the rich nations and the poor nations, and how corruption and warmongering (eg. in Africa) worsens suffering within their own country, and how corruption of other forms within rich nations like the USA are harming not just themselves, but the world in general.).
You hear all that 'AdamKhoo' nonsense about "passive income"? The anti-ethical flawed idea goes, "if you're an intelligent, successful person in today's society, it means you would start multiple passive-income streams, which should eventually overtake your active income. Then you can retire, and your money will just grow without you having to work!" Sure, sounds great. But wait, if this works for everyone, that means everyone will no longer need to work! But wait a min, if everyone's a millionaire and nobody needs to work (because of this wonderful 'passive income'), then who will work to grow food to feed humanity? Who will work to improve technology and the lives of others on the planet?
This entire idea (actually, the entire financial system employed by humanity) is flawed at best, and downright selfish and unethical at worst, because some people would say, "Oh, you're right that passive income can't work if everyone succeeds in using it. But here's the thing : I don't want everyone to succeed in using it. I just want myself to succeed in using it. Everyone else can go on doing whatever hard labour work needs to be done on the planet, on my behalf. As long as I'm not at the bottom, as long as I'm on top of the pack, everything is ok, everything is all correct. I'm cool with survival-of-the-fittest system, as long as I'm the fittest. Screw everyone else to hell. This is how it should be. Heehee!."
Okaaaaay. Here's the thing : it's clear to me that humanity and planet Earth could be a lot more, a lot better, than how it currently is. Maybe it still could be, someday. It's all a matter of choice, individual and collective.
But to be fair, things are certainly not all that bad. I just wanted to get the above off my chest. There is hope for change (Obama isn't a world saviour, but he's a decent human being doing his darnest best in a near-hopeless international political quagmire, a cesspool of corruption...) oops, as I was saying, things are not all bad. There are certainly many ethical, helpful, evolving people everywhere on the planet, just about as many as there are unethical, selfish, decadent people everywhere on the planet. So it's a collective game, a collective tug-of-war, a collective party on Earth. Each side, each individual, each group, harbouring and working out their own agenda (Bilderberg Group, anyone?), whether it be ethical or unethical, religious or secular, world-domination or world-assistantiality.
While tis not exactly a fun-and-games place to be on, planet Earth. But it is a powerful place to be, a powerful time to live in. With great power, lies great opportunities for great good or great harm. With currently accelerating technological progress, there can be much possibilites for the future. The future of our planet, the future of humanity.
Regarding Qn 4 :
Yes, while it's true what you say that because the molarity of the protons or the acids are not given, it's technically not a fair question (since theoretically, a concentrated solution of a weak acid can indeed have a lower pH than a dilute solution of a strong acid).
However, such questions do indirectly imply (meaning it is fair enough to expect students to think this way) that the different acids present (strong versus weak) are probably of similar molarities, so that the question can be fairly answered. In other words, the point of the question, is to test for the students understanding of 'strong' versus 'weak' acids, assuming similar molarities. This is moreso the case at 'O' levels, than at 'A' levels.
So for that question 4, the 'correct' O Level answer would be, in increasing pH :
Nitric acid (stong acid), Vinegar (weak acid), Sugar Solution (neutral), Limewater (alkaline).
You're welcome again, gohby, always glad to share the joy of Chemistry around. May you keep enjoying Chemistry (and Life) and sharing your own joy and love (of Chemistry, of Life) around, too. 
Originally posted by gohby:
Thanks a lot UltimaOnline - your explanation is really thorough and informative! :)
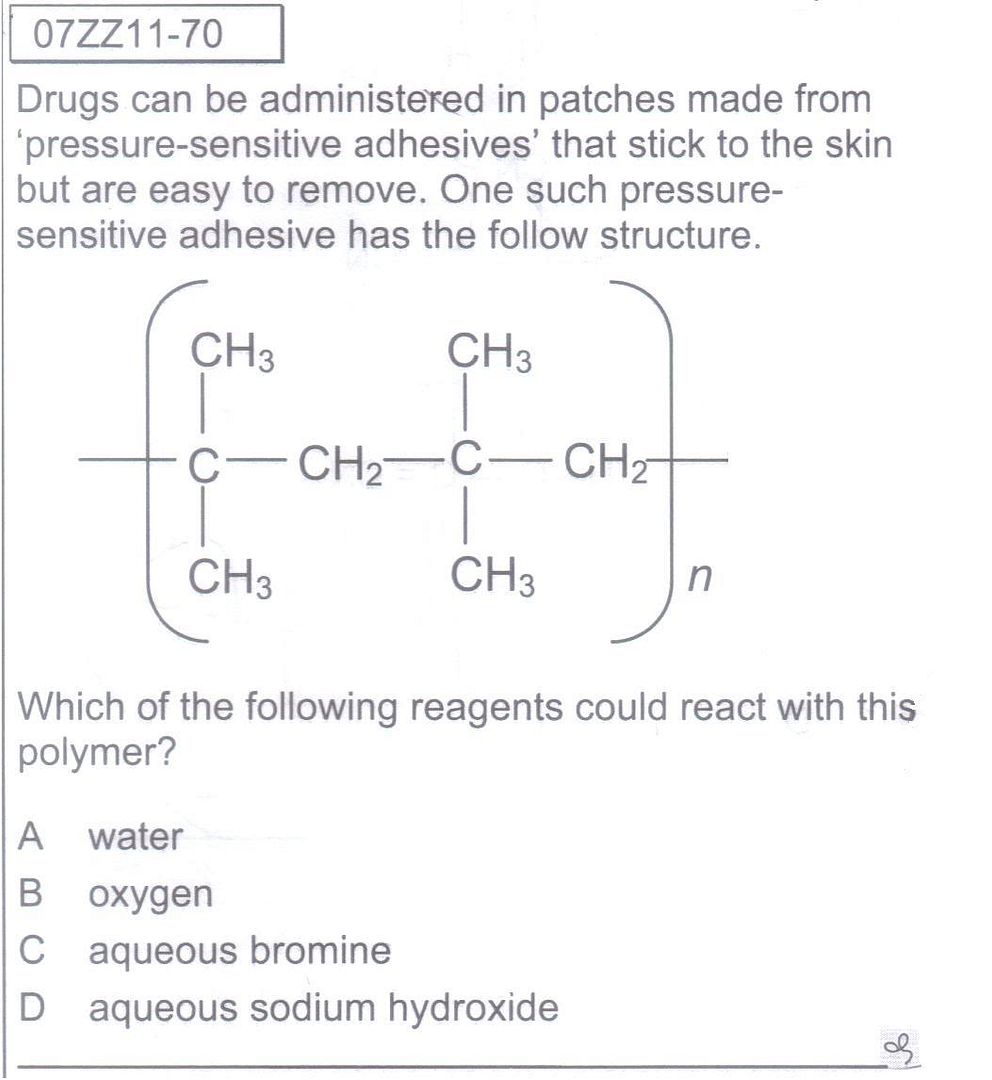
The complexity of Question 1 alarmed me actually. How can a sec sch student, given the materials in the textbook that he/she has been exposed to, know that Al has a significantly oxidation potential? A sec sch student would only be cognisant of the scale of reactivity of metals, and even so, there isn't any clear "cut-off" point to say which metals will oxidise with ease. They will not understand what is oxidation potential, or have access to A Level Chemistry Formulae Booklet either. Besides, they are supposed to conjure that the electrons expelled from the oxidation of aluminium are used to thoroughly reduce NO3- right up to NH4+, even without the presence of a known reducing agent. (I know metals are reducing agents, but what I'm referring to are "special" reducing agents like potassium iodide, etc). What I am more concerned is how the student is able to obtain the answer by establishing the link between whatever that is expressedly taught in the syllabus to the question - in this case, the link, for sec sch students, imo, is very difficult to establish. Is there another way of looking at this question from a perspective of a sec sch student by relating to the stuff in the textbook such that this question doesn't seem so out-of-the-world and chim?
For question 3, since the concentration of hydrogen peroxide in Expt I & II are the same, shouldn't the rate of reaction be the same? In that case, even option A would not be a right choice as it shows the rate of reaction for Expt I is greater than that of Expt II.. Besides, I think it's not very fair for the students if the setter were to truncate the graphs expressing any hints that it's truncated in the qn.. I'm of the opinion that this question is void and there isn't a valid answer though.. Would you agree?
Oh yes, for question 4, given the fact that pH=-lg[H+], can I say it's not possible to compare the pH of vinegar (which is made up of acetic acid/ethanoic acid) and the pH of nitric acid since the concentration of H+ ions is not stated?
Thanks a lot once again!