Oxidation of diol qn
-
Hi, a quick question here:
I have HOCHâ‚‚CHâ‚‚OH.
When it is heated with acidified potassium manganate (VII), the solution was decolourised with effervescence of a colourless gas.
According to the answer scheme, it describes the above rxn as "Oxidation with acidified KMnOâ‚„ to form carbon dioxide.
What I find perplexing is: where did the COâ‚‚ come from?

Many thanks!

-
Originally posted by gohby:
Hi, a quick question here:
I have HOCHâ‚‚CHâ‚‚OH.
When it is heated with acidified potassium manganate (VII), the solution was decolourised with effervescence of a colourless gas.
According to the answer scheme, it describes the above rxn as "Oxidation with acidified KMnOâ‚„ to form carbon dioxide.
What I find perplexing is: where did the COâ‚‚ come from?

Many thanks!

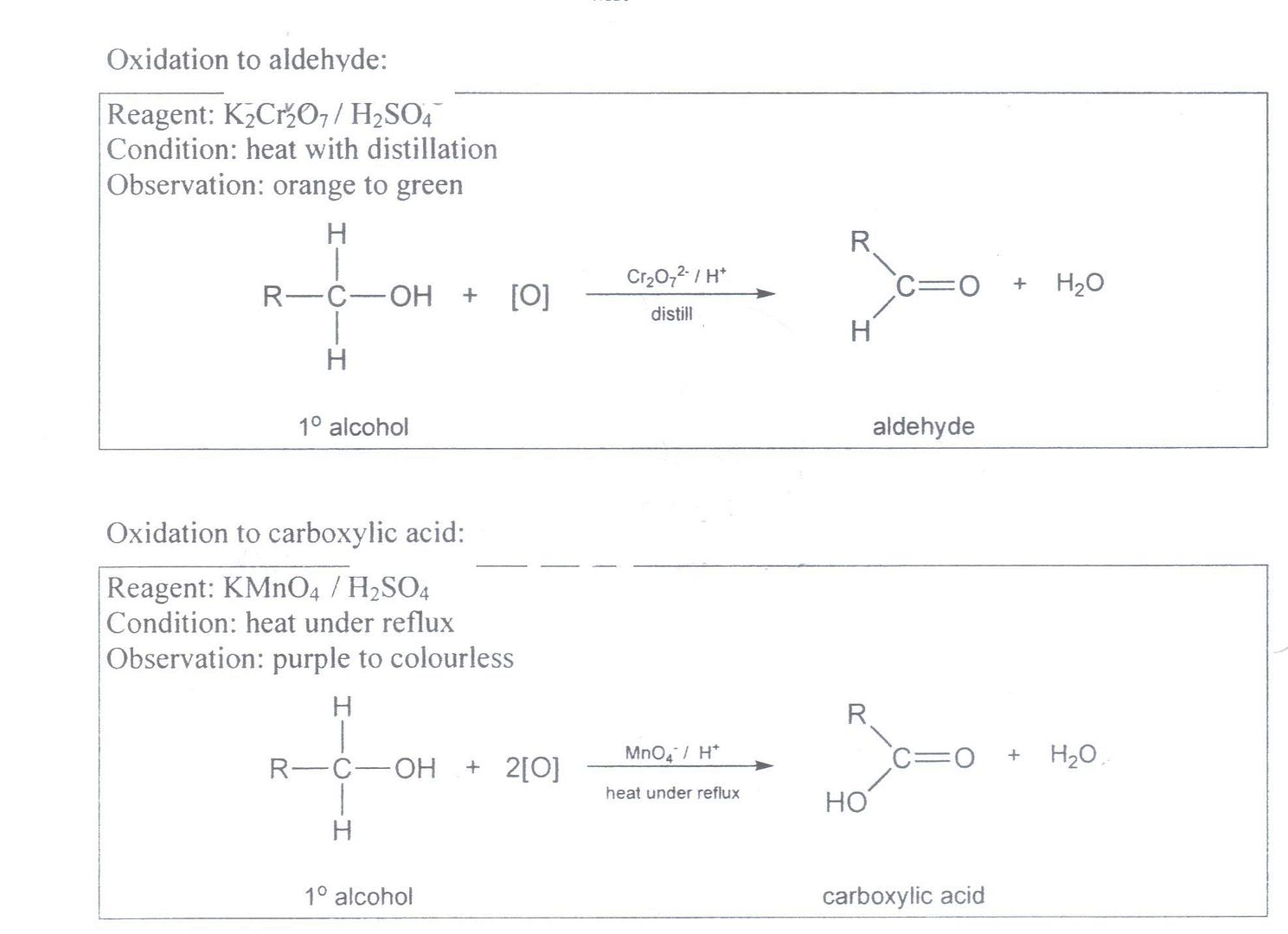
It is part of the 'A' level syllabus (both H2 and H1) requirement that you are aware that ethan-1,2-diol, ethandial, ethandioc acid, and ethandioate ions will all be oxidized by KMnO4 to carbon dioxide and water (while K2Cr2O7 will only oxidize these up to the carboxylic acid level).
-
Originally posted by UltimaOnline:
It is part of the 'A' level syllabus (both H2 and H1) requirement that you are aware that ethan-1,2-diol, ethandial, ethandioc acid, and ethandioate ions will all be oxidized by KMnO4 to carbon dioxide and water (while K2Cr2O7 will only oxidize these up to the carboxylic acid level).
Bearing in mind that potassium manganate (VII) is a stronger oxidising agent than K2Cr2O7, isn't the 'highest' level that those chemicals that you've mentioned can be oxidised is carboxylic acid?
As stated in the notes, heating an alcohol with reflux only yields carboxylic acid (and not further oxidised to carbon dioxide). I'm not doubting the veracity of your explanation, but I just wanna reconcile these seemingly contradictory logics, and the chemistry behind it. :)
-
For normal alcohols and aldehydes, yes indeed, carboxylic acids are the highest level for normal oxidation by both K2Cr2O7 and KMnO4.
But methanol, methanal, methanoic acid and methanoate ion, as well as ethan-1,2-diol, ethandial, ethandioc acid, and ethandioate ions; these 8 compounds are a special category that you should be aware of, that are able to be oxidized to CO2, by KMnO4. The reason is their unusually high C to O ratio (compared to say, ethanol, ethanal and ethanoic acid), which makes them readily supsceptible to further oxidation to CO2 (but only by KMnO4 and not K2Cr2O7).
Two other categories of compounds that KMnO4 and K2Cr2O7 behave differently with, are :
Alkyl benzenes and Alkenes.
For alkyl benzenes, KMnO4 is able to oxidize these to benzoic acid (provided the C atom of the alkyl group just outside the benzene ring is directly bonded to at least one H atom).
For alkenes, KMnO4 at low temperatures will oxidize them to diols, at high temperatures will carry out oxidative cleavage of the alkene double bond.
Originally posted by gohby:Bearing in mind that potassium manganate (VII) is a stronger oxidising agent than K2Cr2O7, isn't the 'highest' level that those chemicals that you've mentioned can be oxidised is carboxylic acid?
As stated in the notes, heating an alcohol with reflux only yields carboxylic acid (and not further oxidised to carbon dioxide). I'm not doubting the veracity of your explanation, but I just wanna reconcile these seemingly contradictory logics, and the chemistry behind it. :)
