Chem A Level Questions
-
Hello, I've encountered some questions in the Chem A Level TYS and would like to seek clarification/explanations from the Chem Pros here on some questions. Any assistance is much appreciated!

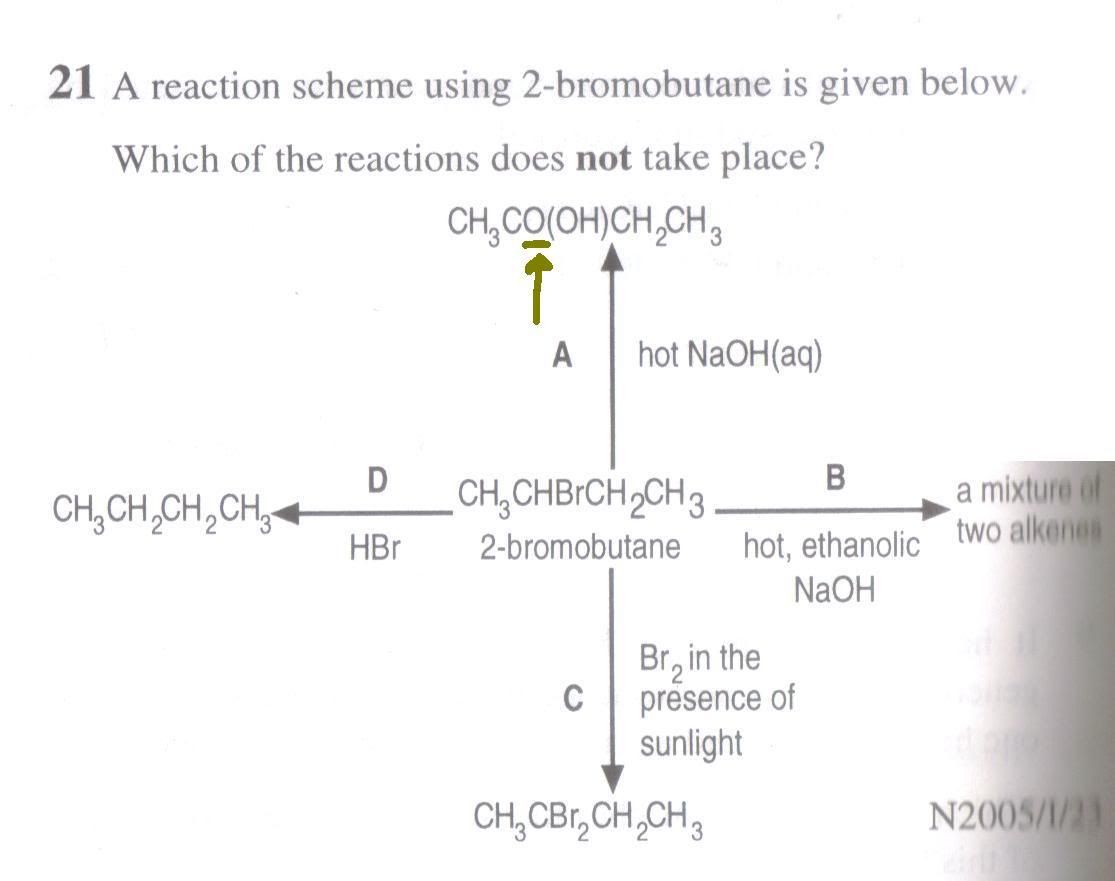
Q1: [N05/I/23] Is there a typo in the TYS publication as highlighted below? For choice A it should have been CH3 CH(OH)CH2CH3 (instead of CH3CO(OH)CH2CH3)?
Also, for choice D, will there be any reaction?
Q2:
In which of these processes is at least one product a gas at room temperature and pressure? [N04/I/24]
A. Dehydration of ethanol
B. Esterification of ethanoic acid by ethanol
C. Oxidation of ethanal by H+/K2Cr2072-
D. Substitution of ethanol by hydrogen bromide
How do I know whether my product is a gas at room temperature? Am I supposed to be cognisant of the boiling points of ethene (in choice A), or the ester in choice B, etc etc?
Q3: [N08/I/38]
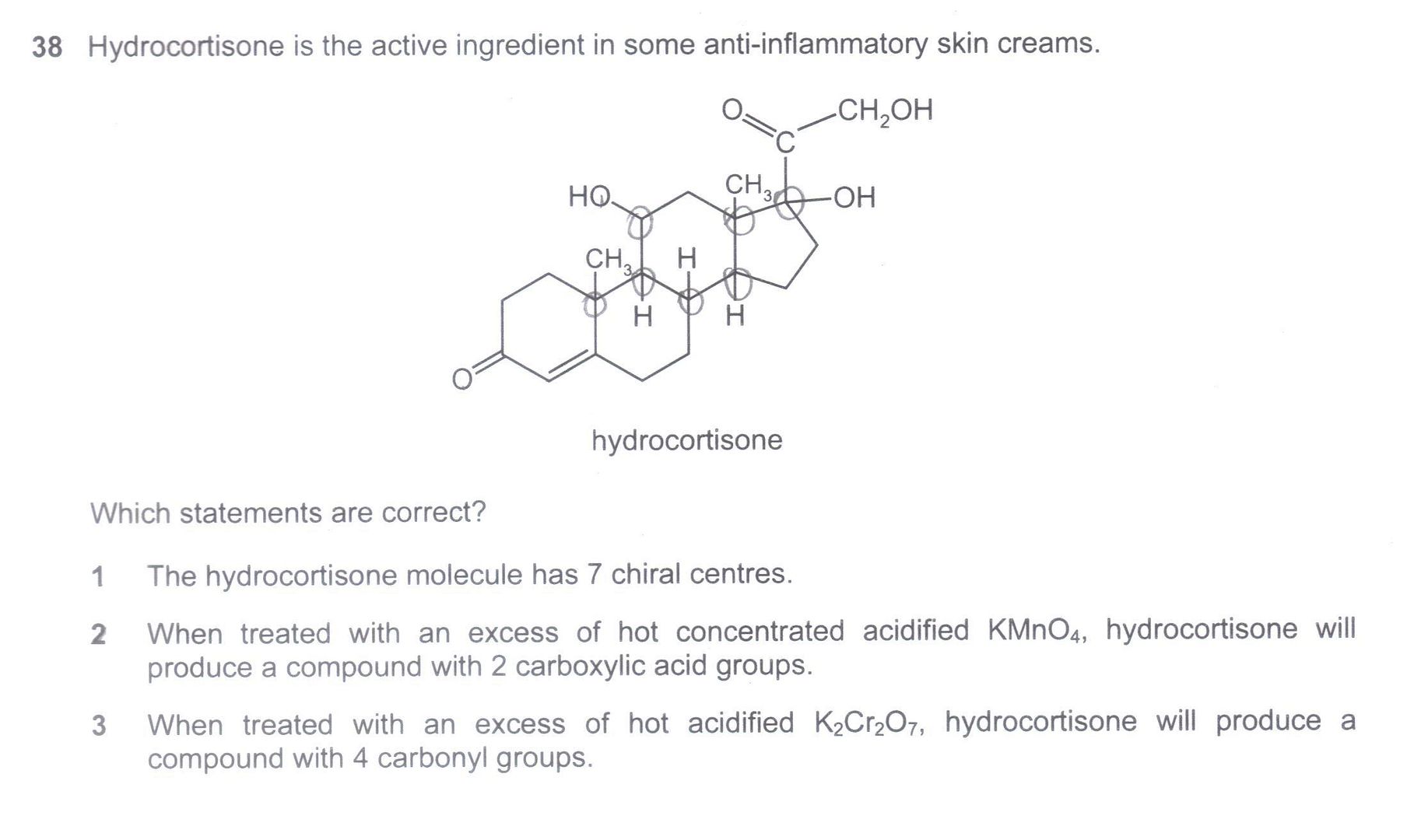

 Choice 1 is correct (Chiral centres circled)
Choice 1 is correct (Chiral centres circled)For Choice 2, I can find 2 carboxylic acid groups, 1 from the oxidative cleavage fo the alkene, and 1 from the oxidation of the terminal alcohol (on the top right hand corner).
For Choice 3, I will have 3 carbonyl groups, 2 from the existing ketones, 1 from the OH being oxidised (in the centre of the diagram). When I oxidise the terminal alcohol on the top right hand corner, I will form a carboxylic acid. Does this carbonyl group from the carboxylic acid count in my sum total?
Q4: [N08/I/20] Modified
When they say Br2 and hv, does hv mean heat+uv?
Q5: [N08/I/24]
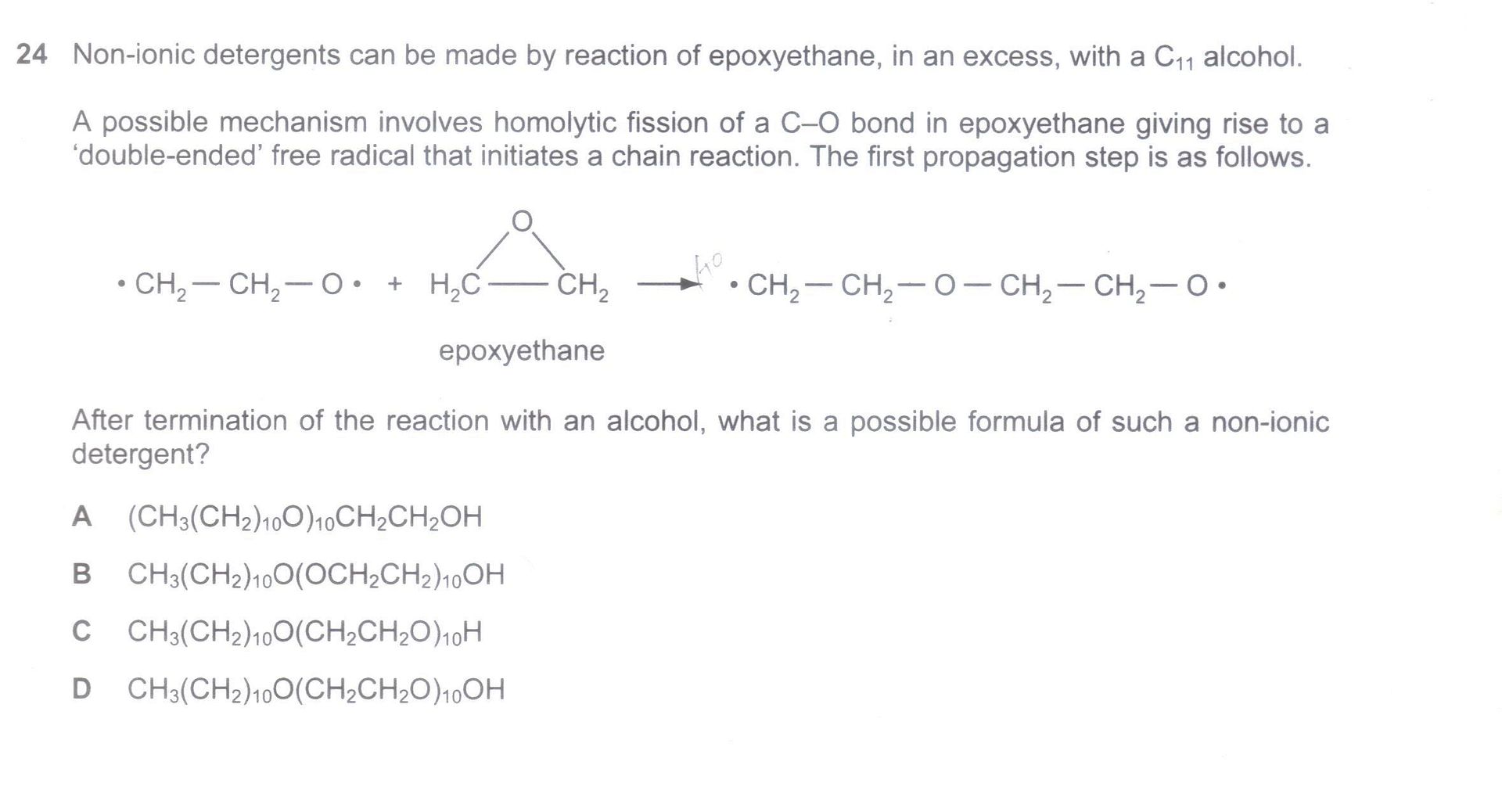
No idea at all..

Q6: [N08/I/20] Modified
What is the effect of adding cold KMnO4 with H3O+ (instead of usual alkaline conditions) to cyclohexene?
Thank you very much for your time! :)
-
Originally posted by gohby:
Hello, I've encountered some questions in the Chem A Level TYS and would like to seek clarification/explanations from the Chem Pros here on some questions. Any assistance is much appreciated!

Q1: [N05/I/23] Is there a typo in the TYS publication as highlighted below? For choice A it should have been CH3 CH(OH)CH2CH3 (instead of CH3CO(OH)CH2CH3)?
Also, for choice D, will there be any reaction?

Q2:
In which of these processes is at least one product a gas at room temperature and pressure? [N04/I/24]
A. Dehydration of ethanol
B. Esterification of ethanoic acid by ethanol
C. Oxidation of ethanal by H+/K2Cr2072-
D. Substitution of ethanol by hydrogen bromide
How do I know whether my product is a gas at room temperature? Am I supposed to be cognisant of the boiling points of ethene (in choice A), or the ester in choice B, etc etc?
Q3: [N08/I/38]


 Choice 1 is correct (Chiral centres circled)
Choice 1 is correct (Chiral centres circled)For Choice 2, I can find 2 carboxylic acid groups, 1 from the oxidative cleavage fo the alkene, and 1 from the oxidation of the terminal alcohol (on the top right hand corner).
For Choice 3, I will have 3 carbonyl groups, 2 from the existing ketones, 1 from the OH being oxidised (in the centre of the diagram). When I oxidise the terminal alcohol on the top right hand corner, I will form a carboxylic acid. Does this carbonyl group from the carboxylic acid count in my sum total?
Q4: [N08/I/20] Modified
When they say Br2 and hv, does hv mean heat+uv?
Q5: [N08/I/24]

No idea at all..

Q6: [N08/I/20] Modified
What is the effect of adding cold KMnO4 with H3O+ (instead of usual alkaline conditions) to cyclohexene?
Thank you very much for your time! :)
Q1. Yes, it's a typo. No, D has no reaction.Q2. Yes, you must know ethene is gaseous at rtp.
Q3. Although carboxylic acid does technically contain the carbonyl group, it is generally accepted that when we say "carbonyl group", we refer specifically only to aldehydes and ketones. Hence answer is 1 & 2 only. But I agree that due to this ambiguity, this is a poorly set (Cambridge 'A' level) question.
Q4. No, hv means irradiation (in this context, irradiation with uv light is implied). High temperatures may provide similar activation energies as uv light, but the symbol hv does not imply heat.
Q5. A killer qn in the 2008 paper, it is also a rather unfair question that is beyond the H2 syllabus, in addition to the fact that the question is ambiguous and poorly phrased. To properly solve this question, you need to be more familiar with mechanisms than the H2 syllabus entails. 'A' level students have no choice but to attempt to solve this problem by pattern recognition alone (ie. without a proper understanding of the mechanism). The best answer would be C, in which the (CH2CH2O)10 is from the excess epoxyethane, and the group at the end should only be a H, not OH (since the front part of the compound already has the O part of the alcohol). It is difficult to explain any further, and I recommend that students skip this question (every year, perhaps increasingly so, Cambridge will add in some similarly unreasonable beyond-syllabus question like this one, but wasting your time trying to fully understand such questions from past exams are not always worth the time or effort, since it won't really help you to answer new types of similarly unreasonable beyond-syllabus questions).
Q6. As long as the temperature is low, ie. in the cold, you will get diol. When the temperature is high, ie. hot KMnO4, you will get oxidative cleavage. (This is due to the fact that higher activation energy is required to progress the multi-step oxidation reaction, of which getting a diol is the first step, and oxidative cleavage is a further step, requiring higher activation energy). Whether it is acidic or alkaline, is not as important as whether it is hot or cold. Again, this point is not often taught in JCs, and therefore here again is another unreasonable question.
-
Hello UltimaOnline, thanks for your prompt and thorough reply!

For Qn2, are students required to know the melting and boiling points of organic compounds, even for esters (that would be required to eliminate choice B)? What are the basic organic compounds that it's assumed that the student will know? For Qn 4, what is the 'full-term' of hv? Is it the SI unit for radiation? What's surprising is googling for hv doesn't give any clear results.. You can imagine the horror when the students saw hv in their A level lol.. For Q5, is the mechanism in question analagous to free radical substitution? Or is it a mechanism that is to be taught in tertiary levels.. Unfortunately, even when one wants to use the 'pattern-spotting' method to solve the question, the pattern is hardly discernable.
-
Originally posted by gohby:
Hello UltimaOnline, thanks for your prompt and thorough reply!

For Qn2, are students required to know the melting and boiling points of organic compounds, even for esters (that would be required to eliminate choice B)? What are the basic organic compounds that it's assumed that the student will know? For Qn 4, what is the 'full-term' of hv? Is it the SI unit for radiation? What's surprising is googling for hv doesn't give any clear results.. You can imagine the horror when the students saw hv in their A level lol.. For Q5, is the mechanism in question analagous to free radical substitution? Or is it a mechanism that is to be taught in tertiary levels.. Unfortunately, even when one wants to use the 'pattern-spotting' method to solve the question, the pattern is hardly discernable.
No, you don't have to memorize the boiling points, but you should be somewhat familiar with the state of the compounds at rtp. Only alkenes and alkanes up to 4 carbons at gaseous at rtp. All esters and alcohols are liquid at rtp, but are nonetheless more volatile (ie. lower boiling point) than water (and you should be able to explain why, in terms of extent of hydrogen bonding).From http://en.wikipedia.org/wiki/Hv :
In physics and chemistry, hν is the formula for a photon's energy in reactions, e.g.
- 2 H2O2 + hν → 2 H2O + O2
- h = Planck's constant (in J·s)
- ν or f = Frequency (in Hz)
- 2 H2O2 + hν → 2 H2O + O2
Yes, Q5 mechanism involves free radicals, but it's rather different from the usual free radical processes studied within the H2 syllabus. Therefore, I advise you to just ignore this question (in the actual 'A' level exam, skip such time-wasting qns and do the rest of the paper first), because it's not worth the time or effort at 'A' levels to try to fully understand this question (and it's unlikely for Cambridge to repeat this type of question, at least for the next couple years).
You're most welcome, gohby

-
Hey guys, I've yet another couple of chem questions and I'm hoping that any chem pros here can help me out..
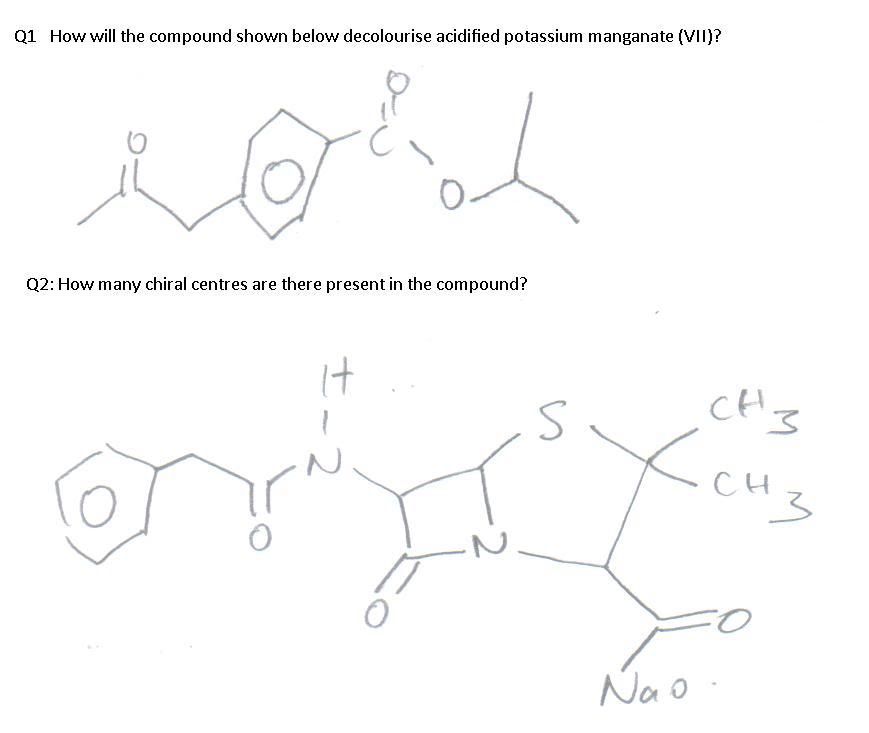
For Q1, I don't see how KMnO4 is going to be decolourised but the answer suggests as such so I want to verify if I've made an oversight.
For Q2, I have counted 3 chiral centres, but the answer suggests 2. (Btw, do I consider counting chiral nitrogens into account)?
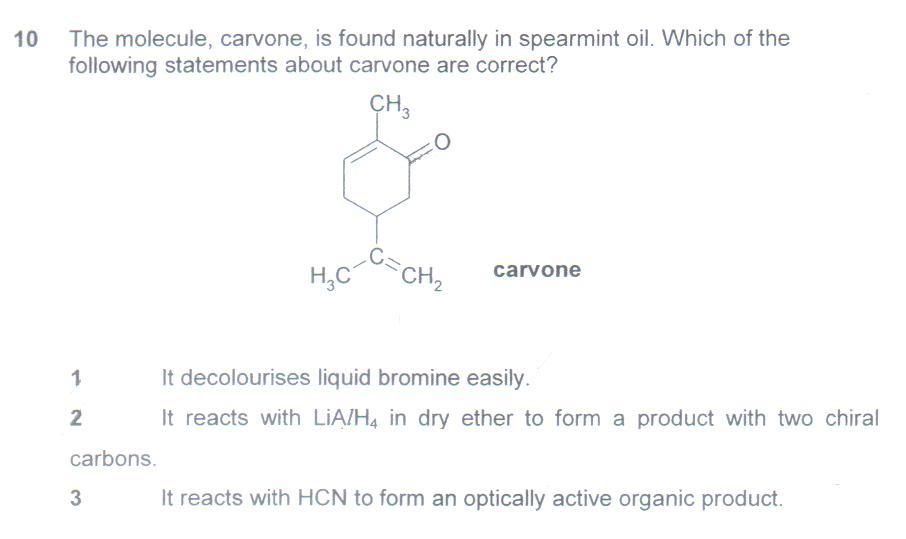
The answer is 1 & 2 but I worked out the answer as 1 & 3 instead.

Q23 - A modified question from N07 A levels. Firstly, what exactly constitutes a CFC? Apprently A is a CFC even though it doesn't contain chlorine or fluorine.. And D is the answer even though it contains fluorine! :S
Another N07 question. Simple question but forgot how to do.. :X
Thank you very much in advance! :D Much appreciated! :)
-
Originally posted by gohby:
Hey guys, I've yet another couple of chem questions and I'm hoping that any chem pros here can help me out..

For Q1, I don't see how KMnO4 is going to be decolourised but the answer suggests as such so I want to verify if I've made an oversight.
For Q2, I have counted 3 chiral centres, but the answer suggests 2. (Btw, do I consider counting chiral nitrogens into account)?

The answer is 1 & 2 but I worked out the answer as 1 & 3 instead.

Q23 - A modified question from N07 A levels. Firstly, what exactly constitutes a CFC? Apprently A is a CFC even though it doesn't contain chlorine or fluorine.. And D is the answer even though it contains fluorine! :S

Another N07 question. Simple question but forgot how to do.. :X
Thank you very much in advance! :D Much appreciated! :)
Q1. The alkyl side chain of the benzene ring on the left will be oxidized, generating a benzoic acid functional group. When heated under reflux with KMnO4 (the requirement to oxidize alkyl side chains of benzene rings in any case), the other side chain will undergo hydrolysis, and subsequently (the products of hydrolysis) oxidation as well.
Q2. Yes there are 3 chiral carbons, 2 in the lactam ring, 1 outside. For H2 Chem, Cambridge will only ask you to count chiral carbons, not chiral nitrogens. There is another difficulty associated with chiral nitrogens, namely nitrogen inversion. See : http://www.rod.beavon.clara.net/chiralit.htm and http://en.wikipedia.org/wiki/Nitrogen_inversion
Q3. 1 and 2 is correct. Option 3 is wrong because the reactant is trigonal planar, and thus the nucleophile can attack from either above or below, and therefore the product is generated as an optically inactive racemic mixture, even though chiral carbons are present. Option 2 is correct because of a new chiral carbon being the alpha carbon of the primary alcohol generated, as well as the preexisting chiral carbon at the base of the hexane ring. Note that LiAlH4 does not reduce alkenes, because both (H- from LiAlH4 and alkenes) are nucleophilic and thus repel each other (ie. they're not gay / homosexual. Nucleophiles are guys who only like electrophiles whom are girls. Only nucleophiles have the 'balls' to attack electrophiles, the pair of 'balls' referring to a lone pair usually, although for benzene nucleophile and alkene nucleophiles a pi bond is used. The 'balls'/lone-pair shoot out to become a bond-pair with the electrophiles/girls, who happily accept them, since they are 'balls'/electron-deficient)
Q4. D is indeed the answer. Don't focus on the 'definition of a CFC'. Focus instead on using a compound in which there are no C-Br or C-Cl bonds, and only C-C, C-H and C-F bonds. C-F bonds do not cleave readily (short bond length = strong bond), and therefore pose much less of a threat to the ozone layer (depleted by free radical substitution reactions), unlike compounds with C-Cl bonds or C-Br bonds which are homolytically cleaved by UV radiation in the stratosphere, generating Cl. or Br. radicals which attack ozone.
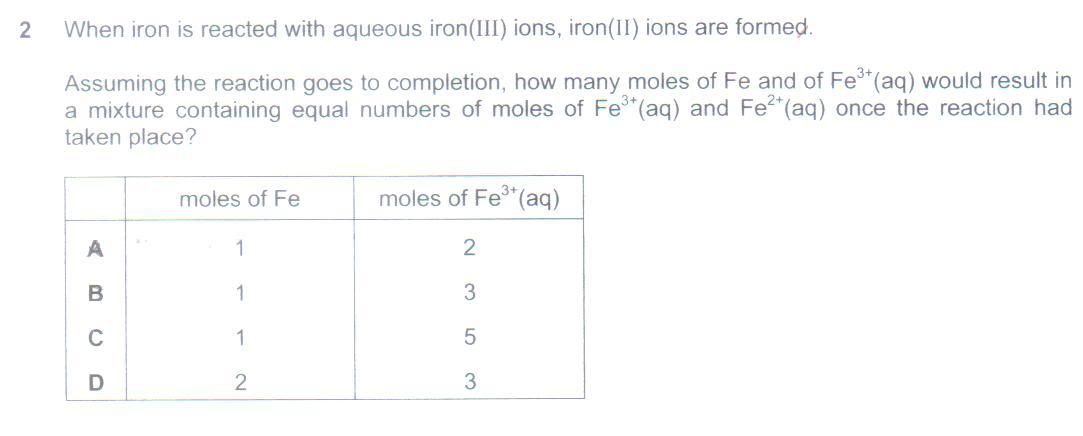
Q5. Do an ICF (Initial Change Final), not ICE (Initial Change Equilibrium) table. Don't use ICE because there isn't any equilibrium to speak of here. Write the balanced redox eqn. Do the ICF table. Since common (chemistry) sense will indicate that Fe is limiting and Fe3+ is in excess, Let the Initial moles of Fe be 1, and Fe3+ be X (remember, your best friend in Chemistry calculations is.... Algebra!), and Fe2+ be 0. Then Change will be -1, -2 and +3 (based on stoichiometry of the balanced redox eqn). Therefore Final will be 0, X-2 and 3. Since at Final, there are equal moles of Fe2+ and Fe3+, hence X-2 = 3, and therefore X = 5. Accordingly, the required mole ratio is 1 Fe : 5 Fe3+.
-
Hi there, I've come across more questions, hope someone could help me out. Much appreciated!

Q1
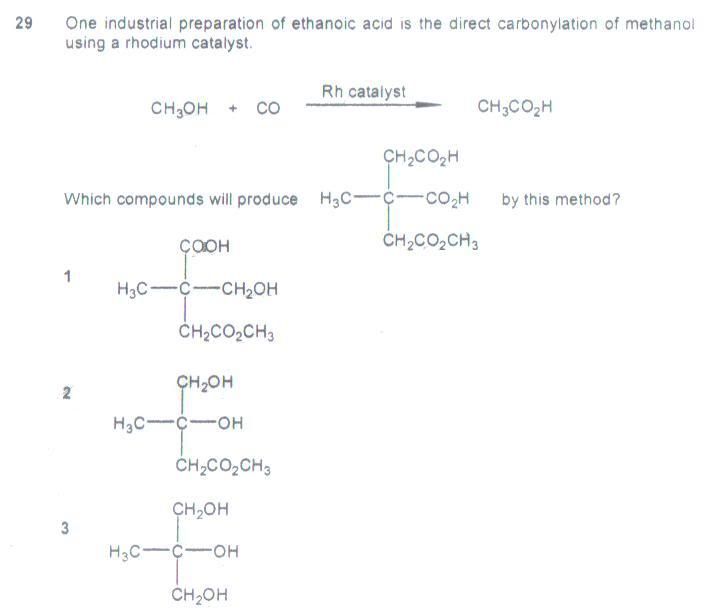
Ans: 1&2
Remark: I couldn’t fathom how, in choice 1, could the CH2OH be directly oxidized to COOH, instead of converting to CH2COOH, which would be analogous to the model. Also, I could not conclusively discard choice 3 as an option. Choice 2 is clear-cut.
Q2
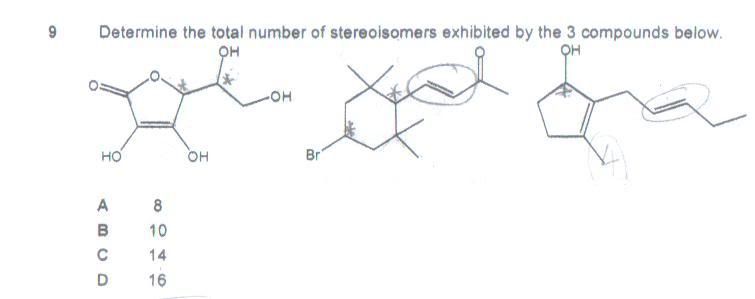
Q3: Which of the following could act as acidic buffers?
1. A 1:2 mixture of HCl and CH3CO2Na
2. A 1:2 mixture of NaHCO3 and Na2CO3
3. A 1:2 mixture of NaOH and NH4Cl
Ans: 1 only.
Remark: An acidic buffer comprises of a weak acid and its salt. In choice 1, I will have CH3CO2Na, CH3CO2Cl and what? (So where is the weak acid and its salt?) What actually transpires in choice 2&3? NaHCO3 seems foreign.
Also, UltimaOnline, sorry to be digging up old questions, but I don't understand the solution put forth for Q5 on the previous post. Could you write down the equation of the reaction? And apparently I don't have the chemistry sense haha, because I don't see how Fe is limiting and Fe3+ is in excess..
Once again, thank you! :)
-gohby -
It's so raw.....
-
Deleted..
-
Originally posted by Darkness_hacker99:
It's so raw.....
Yeah! Cause the formatting screwed up when I copied and pasted from Microsoft Word.
-
Originally posted by chisinau:
Hi there, I've come across more questions, hope someone could help me out. Much appreciated!

Q1

Ans: 1&2
Remark: I couldn’t fathom how, in choice 1, could the CH2OH be directly oxidized to COOH, instead of converting to CH2COOH, which would be analogous to the model. Also, I could not conclusively discard choice 3 as an option. Choice 2 is clear-cut.
Q2

Q3: Which of the following could act as acidic buffers?
1. A 1:2 mixture of HCl and CH3CO2Na
2. A 1:2 mixture of NaHCO3 and Na2CO3
3. A 1:2 mixture of NaOH and NH4Cl
Ans: 1 only.
Remark: An acidic buffer comprises of a weak acid and its salt. In choice 1, I will have CH3CO2Na, CH3CO2Cl and what? (So where is the weak acid and its salt?) What actually transpires in choice 2&3? NaHCO3 seems foreign.
Also, UltimaOnline, sorry to be digging up old questions, but I don't understand the solution put forth for Q5 on the previous post. Could you write down the equation of the reaction? And apparently I don't have the chemistry sense haha, because I don't see how Fe is limiting and Fe3+ is in excess..
Once again, thank you! :)
-gohby
Yo gohby, why the new nick?

Qn1. Tis a guai-lan trick qn. Recognize that single bonds are freely rotatable.

Qn2. The qn is ambiguous (many JCs' prelim exam questions, and sometimes even Cambridge exam qns, relating to isomerism and stereoisomerism are often ambiguous), and furthermore all of the given options are arguably wrong.
Let's look at the 2nd molecule : the two chiral carbons you've marked out, are actually achiral (ie. not chiral). This is because 2 of the 4 groups bonded to those C atoms, are the same (do a trace route). However, this cyclocompound does have geometric isomerism within the ring, because the single bonds of cyclocompounds (as with alkene double bonds) cannot rotate freely. However, this isn't taught within the H2 syllabus, so is this a fair qn?
Ignore this question, and in the exams, skip all ambiguous and/or time-consuming qns first, else you won't have time to complete the paper.
Qn3. The HCl will be totally used up, leaving equal molarities of ethanoic acid and sodium ethanoate. This is indeed an acidic buffer.
For options 2 and 3, these are basic buffers being generated (because these are primarily a weak base, with its conjugate acid). Option 2 is the trickiest option, because NaHCO3 can be considered both a weak acid, and a weak base. However, because Na2CO3 is a significantly stronger base (than NaHCO3 is either basic or acidic), thus this is best considered a basic buffer.
For such questions, you'll have to (mentally, or on paper) do an ICF table : Initial, Change, Final. (This is slightly different from an ICE table, Initial, Change, Equilibrium). See how the given Initial reactants react with each other, work out the Change based on limiting reactant, and see whether the Final products qualify as buffers.
>>> Also, UltimaOnline, sorry to be digging up old questions, but I don't understand the solution put forth for Q5 on the previous post. Could you write down the equation of the reaction? And apparently I don't have the chemistry sense haha, because I don't see how Fe is limiting and Fe3+ is in excess.. <<<
Because that question implied that all the Fe is used up at the end of the experiment, when there are "equal moles of Fe2+ and Fe3+". Since this is not an equilibria qn, it implies that there are no more Fe present (or else any Fe still present would be used up to react with any Fe3+ still remaining to form Fe2+). Therefore, Fe can be deduced to be the limiting reactant. Accordingly, do an ICF table, and let the Initial moles of Fe and Fe3+ be 1 : x. Change will be (according to stoichiometry) -1, -2, +3. Final will be 0, x-2 and 3. Since x-2 = 3, this implies x = 5, and therefore the answer is 1 Fe : 5 Fe3+.