electrochemistry
-
How do you choose what reactions to use, when it involves water?
From what I know, equations with H+ are chosen when the reaction occurs in an acidic medium, while equations with OH- are chosen when it occurs in an alkaline medium.
So, in acidic solution, and assuming H2O is oxidised,
H20 -> H2O2 + 2H+ + 2e- and
H2O -> O2 + 4H+ + 4e-
and in alkaline solution, assuming H2O is reduced,
O2 + 2H2O + 4e- -> 4OH- and
2H2O + 2e- -> H2 + 2OH-
What's the basis for selection? Electrode potential such that Ecell will be maximum/minimum? Thanks in advance.
-
Originally posted by donkhead333:
How do you choose what reactions to use, when it involves water?
From what I know, equations with H+ are chosen when the reaction occurs in an acidic medium, while equations with OH- are chosen when it occurs in an alkaline medium.
So, in acidic solution, and assuming H2O is oxidised,
H20 -> H2O2 + 2H+ + 2e- and
H2O -> O2 + 4H+ + 4e-
and in alkaline solution, assuming H2O is reduced,
O2 + 2H2O + 4e- -> 4OH- and
2H2O + 2e- -> H2 + 2OH-
What's the basis for selection? Electrode potential such that Ecell will be maximum/minimum? Thanks in advance.
It is the protons from water which are reduced, and it is the hydroxide ions from water which is oxidized.
Do not use reactions that generate H2O2, unless you're specifically told to do so in the question (most usually, H2O2 will be a reactant, not a product, ie. use the right to left equation instead of left to right).
Notice that in your equation O2 + 2H2O + 4e- -> 4OH-, it is not water that is reduced, but molecular dioxygen (ie. O2 gas) which is reduced.
Again, unless the question specifically dictates molecular dioxygen (ie. O2 gas) being reduced at the cathode, do not use that equation. Use the one in which (the protons from) water are reduced to molecular dihydrogen (ie. H2 gas) instead.
For electrolytic cells, you need not worry about the cell potential, because the source of the electricity comes from elsewhere (eg. the wall socket). Therefore, you need not worry about the feasibility of whether you should use water or protons as the reactant to be reduced @ cathode, or whether you should use water or hydroxide ions as the reactants to be oxidized @ anode. Cambridge will accept either choice of reactants, for both.
-



-



-
Hello!
I have a question for UltimaOnline. It is not related to this topic bit is related to A-level chemistry. May I ask?
-
Originally posted by Kahynickel:
Hello!
I have a question for UltimaOnline. It is not related to this topic bit is related to A-level chemistry. May I ask?
Sure. If it's another topic on A level Chem, you can feel free to make a new forum thread, eg. titled "[H2 Chemistry] - Ionic Equilibria" or "[A level Chemistry] - Kinetics", etc. -
I am an A-level teacher from Karachi, Pakistan. I recently viewed a question paper of Nov 2009/P3 (free response questions). It was not available for download. Nonetheless i wrote all the contents and then let my students solve that which they did.
Now I require all the past papers of A-level from 2002-2010. I have searched the internet but found no site which has this stuff.
I require them right now because the new session for Nov students is about to start. I have past papers from CIE but the papers from Singapore are different. How can i get these papers?
-
Originally posted by Kahynickel:
I am an A-level teacher from Karachi, Pakistan. I recently viewed a question paper of Nov 2009/P3 (free response questions). It was not available for download. Nonetheless i wrote all the contents and then let my students solve that which they did.
Now I require all the past papers of A-level from 2002-2010. I have searched the internet but found no site which has this stuff.
I require them right now because the new session for Nov students is about to start. I have past papers from CIE but the papers from Singapore are different. How can i get these papers?
You'll have to email each of the 7 publishers listed on this website, explain to them your situation and location, and ask them if they'll sell you the exam paper compilation book (known as a "Ten Year Series") through use of your credit card, and ask them whether they're willing to ship to your location in Pakistan.http://www.seab.gov.sg/pastYearPaperPublishers.html
-
Thank you very much for the list. i have mailed all of them.
Are past papers also available in CD?
-
Hello, I have 2 electrochem qns here:
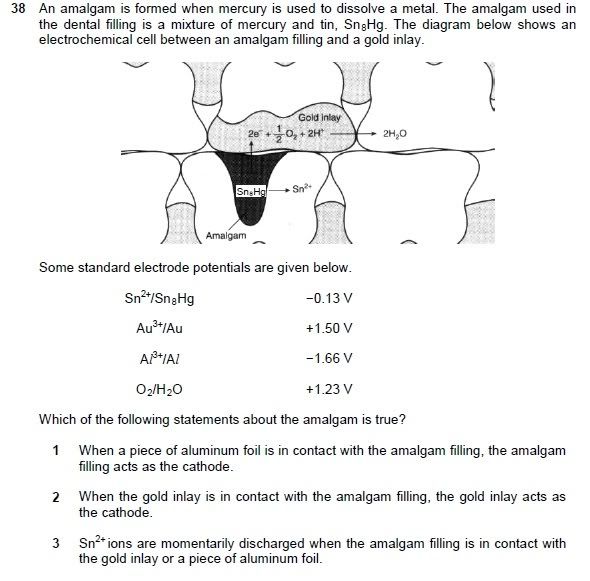
Q38: I understand that 1 & 2 are correct given the reduction potentials, but why is 3 wrong?
No idea on what is going on. :S
Relevant E0 values:
Fe3+ + e- --> Fe2+ +0.77V
MnO4- + 8H+ + 5e- --> Mn2+ + 4H20 +1.52V
Thank you very much! :)
-
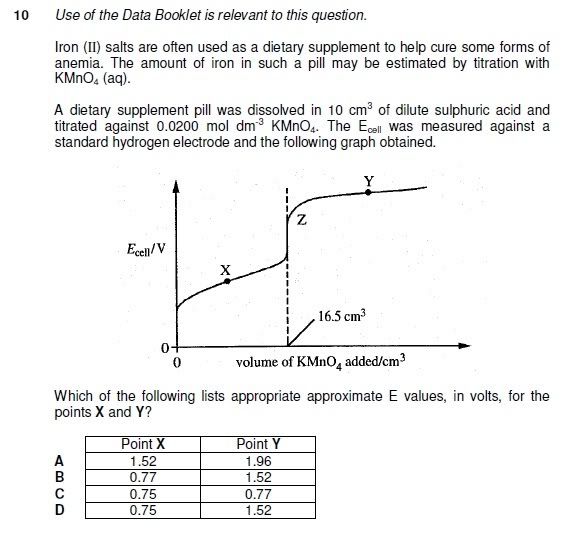
FOR 10
The answer is option B.
Remember that acidified KMnO4 is being added from the burrette. Before reaching the end-point at 16.5 cm3 that is at point X its amount or moles are less than that Fe2+ which is present in larger amount than KMnO4. Therefore technically speaking the whole redox system will have an standard electrode potential value of less than +1.52V. Now at pooint X Fe2+ is at higher concentration therefore at this point the value will be +0.77V.
Now at Z the concentartion of Fe2+ is effectively nil. All Fe2+ has been turned into Fe3+. On the other hand the despite reaching the end-point at 16.5cm3 acidified KMnO4 is still being added therefore its concentration is increasing. don't forget there is no Fe2+ now but a handful of MnO4- so the value at point Y is equal to the value of standard electrode potential of acidified manganate (VII) which is +1.52V.
(Although the concentration does affect the value of E. In this question the [MnO4] is well below 1 molar so I don't think that it will be +1.52V but the options are somewhat else. So this explanation is ample).
-
Kahynickel has already helped you explain the redox-titration qn.
As for the electrochem qn, the key lies in understanding the word "discharge". In medical terms regarding bodily fluids, it simply means "to release" or "to expel".
In chemistry terms, it means "to remove a charge". Therefore in regard to Sn2+ ions, it means to REDUCE Sn2+. But based on the data given in the question, Sn is being OXIDIZED to Sn2+. Therefore, for option 3 to be correct, the word "discharged" should be replaced with the word "generated".
Originally posted by gohby:Hello, I have 2 electrochem qns here:

Q38: I understand that 1 & 2 are correct given the reduction potentials, but why is 3 wrong?

No idea on what is going on. :S
Relevant E0 values:
Fe3+ + e- --> Fe2+ +0.77V
MnO4- + 8H+ + 5e- --> Mn2+ + 4H20 +1.52V
Thank you very much! :)