A Level Transition Elements Syllabus
-
Hello,
I would like to enquire what exactly is in the syllabus for H2 9647 Chemistry, transition elements.
1. Are students required to know the transition elements complexes and their colours? If so, which ones?
Those that I memorised as a student are [Cu(H2O)6]2+ - light blue, [Cu(NH3)4(H2O)2]2+ - dark blue, SCN- complexes are blood red. Apparently these doesn't suffice?
2. Know the relative strengths of different ligands or explaining why are certain transition elements compounds of a certain colour (Due to weak/strong ligand--> Difference in absorption corresponding to the wavelength and energy --> Difference in the colour that is reflected.)
However, such concepts are quite heavily dwelled on for some schools, leaving students even more befuddled.
As taken from:
http://www.seab.gov.sg/aLevel/2012Syllabus/9647_2012.pdf
Under the topic on transition elements,
(h)
(i) explain the reactions of transition elements with ligands to form complexes, including the complexes of copper(II) ions with water and ammonia
(ii) describe the formation, and state the colour of, these complexes(l)
explain, in terms of d orbital splitting, why transition element complexes are usually coloured
From the syllabus, there seems to be no mention that these ceoncepts are required. It would be good for the interest of students to know what is in their syllabus and what's not lest they try to figure out / spend unnecessary time memorising stuff that are out of their syllabus when they have many concepts that are WITHIN their syllabus which they have to grapple with.
It would be good if the air is cleared. :)
On this note,
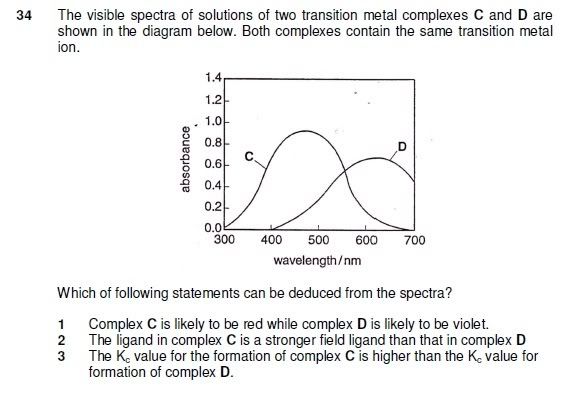
Here is a qn which actually appeared in prelims that leverages on the knowledge on strong/weak ligands & their wavelength absorbed, etc.
1 & 2 are logically deducable but how is Kc related to this question?
Many thanks for any help/advice given! =)
Cheers
-
Hi Gohby,
I recall we've had this conversation before. While I do empathize and sympathize with your feeling of injustice for students (both your own students as well as the student population in general), but it's a bit of a vicious catch-22 cycle : because JCs / the education system are survival-of-the-fittest kiasu and every year teach students more and more beyond the syllabus, consequently in order to make the examinations more discriminating, Cambridge feels obligated to test further and further beyond the syllabus, at least unofficially. Officially, everyone will deny everything.
Transition metal complexes are a touchy topic, and because much of this topic is rather complex (pun intended) beyond the H2 syllabus, it's no wonder that this topic is a potential source for many killer (and in your perspective : unfair) exam qns.
The syllabus is deliberately vague, with no mention of many associated concepts that many JCs (out of kiasuism) teach, and that both internal JC exams as well as Cambridge exams may (directly or indirectly) examine on.
Yes, you're right that the three complex ions and their colours you memorized when you were a student yourself, does not suffice. But then again, MOE-SEAB and Cambridge (deliberately, by intention) did not release any official list of complex ion colours for A level students to memorize. So many JC students, in typical Singapore kiasu pattern, desperately try to obtain as many different 'top' JC notes as possible, to memorize as many obscure complex ion colours as possible, to cover themselves.
Unfair? In that sense, probably. But on the flip side, MOE-SEAB / Cambridge will argue (fairly enough) that if the student has truly understood the concepts, he/she would be able to deduce the probable colours and/or geometries, and would still be able to be atop the bell-curve and score a distinction.
In that regard, advise your students not to worry too much about needing to be too kiasu and memorize all the colours and geometries of all the obscure complex ions in the world. That's not necessary nor helpful. Sure, internal JC exams and once in a while, even Cambridge, may ask such beyond-the-syllabus questions, but the student who wisely focuses on truly understanding the underlying concepts will more than make up for that single lost mark, and still come out ahead and on top of the bell-curve.
On the the chemistry content.
Strong ligands are not the same as strong field ligands.
Weak ligands are not the same as weak field ligands.
The former has to do with availability of lone pairs, nucleophilicity and basicity of the ligands, and the consequent strength of the coordinate dative bond generated. The latter has to do with the magnitude of energy difference of the d orbitals upon splitting, which will have a direct impact on the resultant colours of the complex ions.
I agree with you that option 3 of the MCQ, should not be included as the correct answer. The setter of the question appears to him/herself have confused the concept of strong-field ligand, with strong ligand. While there is some correlation to the two, they are not the same, and therefore option 3 will not necessarily be true.
Originally posted by gohby:Hello,
I would like to enquire what exactly is in the syllabus for H2 9647 Chemistry, transition elements.
1. Are students required to know the transition elements complexes and their colours? If so, which ones?
Those that I memorised as a student are [Cu(H2O)6]2+ - light blue, [Cu(NH3)4(H2O)2]2+ - dark blue, SCN- complexes are blood red. Apparently these doesn't suffice?
2. Know the relative strengths of different ligands or explaining why are certain transition elements compounds of a certain colour (Due to weak/strong ligand--> Difference in absorption corresponding to the wavelength and energy --> Difference in the colour that is reflected.)
However, such concepts are quite heavily dwelled on for some schools, leaving students even more befuddled.
As taken from:
http://www.seab.gov.sg/aLevel/2012Syllabus/9647_2012.pdf
Under the topic on transition elements,
(h)
(i) explain the reactions of transition elements with ligands to form complexes, including the complexes of copper(II) ions with water and ammonia
(ii) describe the formation, and state the colour of, these complexes(l)
explain, in terms of d orbital splitting, why transition element complexes are usually coloured
From the syllabus, there seems to be no mention that these ceoncepts are required. It would be good for the interest of students to know what is in their syllabus and what's not lest they try to figure out / spend unnecessary time memorising stuff that are out of their syllabus when they have many concepts that are WITHIN their syllabus which they have to grapple with.
It would be good if the air is cleared. :)
On this note,

Here is a qn which actually appeared in prelims that leverages on the knowledge on strong/weak ligands & their wavelength absorbed, etc.
1 & 2 are logically deducable but how is Kc related to this question?
Many thanks for any help/advice given! =)
Cheers
-
To UltimaOnline
you mentioned the following generalization regrading the spliting pattern:
Strong ligands are not the same as strong field ligands.
Weak ligands are not the same as weak field ligands
Would you please elaborate it some examples?
-
Originally posted by hoay:
To UltimaOnline
you mentioned the following generalization regrading the spliting pattern:
Strong ligands are not the same as strong field ligands.
Weak ligands are not the same as weak field ligands
Would you please elaborate it some examples?
First of all, this aspect is totally beyond the H2 Chemistry syllabus. And even it's unlikely, even when Cambridge on occasion go beyond the syllabus in their questions, to ask directly on this. In other words, H2 Chem students really needn't concern themselves regarding this.As an example, consider the water molecule versus the hydroxide ion, as ligands. Obviously, the hydroxide ion is a far stronger ligand, because it's a lot more electron-rich (the O atom in OH- has a negative formal charge, while the O atom in H2O only has a partial negative charge), and therefore it's a far stronger ligand (and nucleophile, and base) compared to the water molecule.
(A Lewis base is a dative bond donor. If you're a Lewis base donating dative bond to a metal ion, you're called a ligand. If you're a Lewis base donating dative bond to a proton, you're called a Bronsted-Lowry base. If you're a Lewis base donating dative bond to an electrophile, you're called a nucleophile. Notice that NH3, functions well as all 3 types of Lewis bases.)
Experimental evidence have shown, however, that when complexed with water ligands, the magnitude of the energy difference of the non-degenerate d orbitals (of the metal ion) after d-d* splitting, is greater than when complexed with hydroxide ligands. In other words, we say that the water ligand is a stronger-field ligand compared to the hydroxide ligand.
What determines whether a ligand is a strong or weak ligand? The strength of a ligand parallels it's basicity and nucleophilicity (even though occassionally for some species, basicity and nucleophilicity do not parallel each other, eg. F- is a stronger base compared to I-, but I- is a stronger nucleophile compared to F-. H2 Chem students should be able to figure out why).
What determines whether a ligand is a strong field or weak field ligand? Several factors, including the specific electron configuration of the ligand, as well as the specific electron configuration of the metal ion. To understand this fully requires a thorough understanding of crytal field theory, ligand field theory and molecular orbital theory, all of which are *way* beyond the H2 syllabus.
http://en.wikipedia.org/wiki/Crystal_field_theory
http://en.wikipedia.org/wiki/Ligand_field_theory
http://en.wikipedia.org/wiki/Molecular_orbital_theory
For those interested (ie. not required for H2 Chem syllabus), the most relevant content has been reproduced below :The size of the gap Δ between the two or more sets of orbitals depends on several factors, including the ligands and geometry of the complex. Some ligands always produce a small value of Δ, while others always give a large splitting. The reasons behind this can be explained by ligand field theory. The spectrochemical series is an empirically-derived list of ligands ordered by the size of the splitting Δ that they produce (small Δ to large Δ; see also this table):
I− < Br− < S2− < SCN− < Cl− < NO3− < N3− < F− < OH− < C2O42− < H2O < NCS− < CH3CN < py < NH3 < en < 2,2'-bipyridine < phen < NO2− < PPh3 < CN− < CO
It is useful to note that the ligands producing the most splitting are those that can engage in metal to ligand back-bonding.
The oxidation state of the metal also contributes to the size of Δ between the high and low energy levels. As the oxidation state increases for a given metal, the magnitude of Δ increases. A V3+ complex will have a larger Δ than a V2+ complex for a given set of ligands, as the difference in charge density allows the ligands to be closer to a V3+ ion than to a V2+ ion. The smaller distance between the ligand and the metal ion results in a larger Δ, because the ligand and metal electrons are closer together and therefore repel more.
-
If you dont know...chances are many others do not either.
Going to an exam requires some intelligence gathering if you are exam-smart.
Already encountered many exams where I did only half the paper but came out knowing I would get at least a B+ if not A and I'm usually right.

Its defeats the spirit of learning, but its exams we are talking about here.
So there's nothing unfair about this or that being tested/untested in exams.
You just have to know your peers and what you are up against in exams.