A Level H2 Chemistry Prelim Questions
-
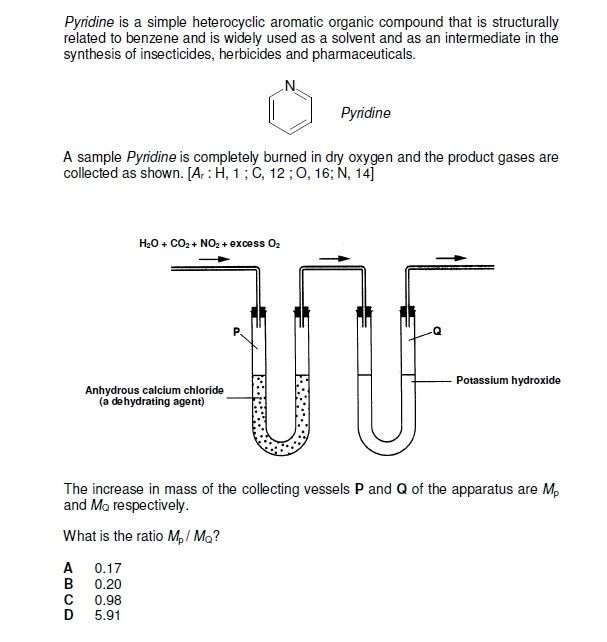
The answer is A. I think it is because water, nitrogen dioxide and oxygen react to form nitric acid, with is absorbed by KOH together with carbon dioxide.
In vessel P, water is absorbed.
Hence answer= 18/44+63.
(That's the only plausible explanation that I can conjure of)
However, since water is required for the formation of nitric acid, why would there be any water left, to be absorbed by Vessel P, in the first place?
-
Originally posted by gohby:

The answer is A. I think it is because water, nitrogen dioxide and oxygen react to form nitric acid, with is absorbed by KOH together with carbon dioxide.
In vessel P, water is absorbed.
Hence answer= 18/44+63.
(That's the only plausible explanation that I can conjure of)
However, since water is required for the formation of nitric acid, why would there be any water left, to be absorbed by Vessel P, in the first place?
You're right in that "water, nitrogen dioxide and oxygen react to form nitric acid", but for the purpose of this question, the question setter has intended for the student to regard that any hydrolysis of nitrogen dioxide before reaching the apparatus is negligible, and that only upon coming into direct contact with the potassium hydroxide, will 'hydrolysis' occur (to be precise : it's not 'hydrolysis' in the sense of 'chemical reaction with water', but it's really a nucleophilic attack by the hydroxide nucleophile) to generate the nitrate ion salt.(Bonus : H2 Chem students should be able to draw the mechanism for the OH- nucleophile attacking the CO2 and NO2 electrophiles, and therefore, based on the mechanism, deduce the identity and structure of the products.)
Similarly for the carbon dioxide. In other words, assume that the two non-metal covalent acidic oxides, will only react with the KOH, and not with the water present initially. Even if the student isn't familiar with or unable to deduce the identity of the final products (of the reaction of CO2 and NO2 with KOH), it does not matter, thanks to the law of conservation of mass.
Now that this assumption is utilized, to solve this question, the student has to balance the equation to apply stoichiometry. Although we do not know the exact moles of the pyridine combusted, but since the final answer is a ratio, it does not matter. Use one mole for the reactant.
Multiply the moles of H2O, CO2 and NO2 generated by their molar masses, to get the ratio of sample masses of all three species : H2O, CO2 and NO2.
Take the ratio accordingly (sample mass of H2O) / (sample mass of CO2 + sample mass of NO2), and you will obtain at the required answer.
-
Ok, got it, thank you very much, UltimaOnline! :)