H2 Chem MCQ qns..
-
1. Below shows the electronic configuration of the three d-block elements in the Periodic Table.
Element X: [Ar]3d^7 4s^2
Element Y: [Ar]3d^8 4s^2
Element Z: [Ar]3d^10 4s^1
Which one of the following statement is incorrect?
A The electronic configuration of central metal ion for [Y(CN)6]4- is [Ar]3d^8
B Upon reduction from ZCl2(aq) to [ZCl2]-(aq), the solution turned colourless.
C The E* value of the X3+/X2+ is less positive than that of Z3+/Z2+
D X is likely to exist as K2X2O7.
Answer is D. I know B and C are out, but I chose A. I thought Y would lose 2 electrons to form Y2+, then forming the complex ion, which has the correct electronic config?
2. 2,2-dimethylpropane reacts with chlorine gas in the presence of ultraviolet light to give a mixture of products. Which one of the following statements is correct regarding this reaction?
A. Only one mono-substituted product is formed.
B. Only the propagation steps involves C-Cl bond formation
C. Only the initation step of the mechanism involves homolytic fission.
D. Both propagation and termination steps produce hydrogen chloride.
Answer is A. I chose C, because I thought the one in propagation step only produces 1 radicals; homolytic fission must produce 2 radicals right? Why is it A anyway? I thought they can be a lot of chlorine substitution going on in one molecule.
3. An organic compound X undergoes the following reactions.
i) It decolourises a solution of bromine in tetreachloromethane.
ii) It reacts with phosphorus pentachloride giving copious white fumes of HCl.
iii) It reacts with hot alkali to give a compound with two alcohol functional groups.
Which compound could be X?
A. HOH2CCH=CHCH=CHCH2Cl
B. Cl2CHCH=CHCH2COOH
C. BrCH2CH2CHClCH2COCl
D. ClCH2CH2Ch=CHCH(Cl)CH2OH
Answer is A. I know C and D are out, but gosh, A and B seems to be both correct!
4. (Choice A- statements 1,2,3 are all corect. B - statement 1,2 are correct. C - statement 2,3 are correct. D - Only statement 1 is correct)
GABA is a neurotransmitter released by red algae which encourages shellfish larvae to settle on the ocean bed.
GABA: H2NCH2CH2CH2CO2H
Which of the following statements are correct?
1. It is a 2-aminocarboxylic acid.
2. It is soluble in water due to zwitterion formation
3. It migrates to the anode of an electrolytic cell at pH2.
Answer is C (statement 2,3). I know 3 is correct, but how can it form a zwitterion like that? I thought there must be a so called alpha carbon, where the NH2 and COOH group must belong to the same carbon atom. What's a aminocarboxylic acid too? :/
sorry for the wall of texts, thanks for your replies man. these are from Vjc 2009 prelim paper.
-
Originally posted by qdtimes2:
1. Below shows the electronic configuration of the three d-block elements in the Periodic Table.
Element X: [Ar]3d^7 4s^2
Element Y: [Ar]3d^8 4s^2
Element Z: [Ar]3d^10 4s^1
Which one of the following statement is incorrect?
A The electronic configuration of central metal ion for [Y(CN)6]4- is [Ar]3d^8
B Upon reduction from ZCl2(aq) to [ZCl2]-(aq), the solution turned colourless.
C The E* value of the X3+/X2+ is less positive than that of Z3+/Z2+
D X is likely to exist as K2X2O7.
Answer is D. I know B and C are out, but I chose A. I thought Y would lose 2 electrons to form Y2+, then forming the complex ion, which has the correct electronic config?
2. 2,2-dimethylpropane reacts with chlorine gas in the presence of ultraviolet light to give a mixture of products. Which one of the following statements is correct regarding this reaction?
A. Only one mono-substituted product is formed.
B. Only the propagation steps involves C-Cl bond formation
C. Only the initation step of the mechanism involves homolytic fission.
D. Both propagation and termination steps produce hydrogen chloride.
Answer is A. I chose C, because I thought the one in propagation step only produces 1 radicals; homolytic fission must produce 2 radicals right? Why is it A anyway? I thought they can be a lot of chlorine substitution going on in one molecule.
3. An organic compound X undergoes the following reactions.
i) It decolourises a solution of bromine in tetreachloromethane.
ii) It reacts with phosphorus pentachloride giving copious white fumes of HCl.
iii) It reacts with hot alkali to give a compound with two alcohol functional groups.
Which compound could be X?
A. HOH2CCH=CHCH=CHCH2Cl
B. Cl2CHCH=CHCH2COOH
C. BrCH2CH2CHClCH2COCl
D. ClCH2CH2Ch=CHCH(Cl)CH2OH
Answer is A. I know C and D are out, but gosh, A and B seems to be both correct!
4. (Choice A- statements 1,2,3 are all corect. B - statement 1,2 are correct. C - statement 2,3 are correct. D - Only statement 1 is correct)
GABA is a neurotransmitter released by red algae which encourages shellfish larvae to settle on the ocean bed.
GABA: H2NCH2CH2CH2CO2H
Which of the following statements are correct?
1. It is a 2-aminocarboxylic acid.
2. It is soluble in water due to zwitterion formation
3. It migrates to the anode of an electrolytic cell at pH2.
Answer is C (statement 2,3). I know 3 is correct, but how can it form a zwitterion like that? I thought there must be a so called alpha carbon, where the NH2 and COOH group must belong to the same carbon atom. What's a aminocarboxylic acid too? :/
sorry for the wall of texts, thanks for your replies man. these are from Vjc 2009 prelim paper.
Q1.
The electron configuration of an uncomplexed ion (ie. without ligands) and a complexed ion (ie. with ligands) is different : dative bonding means the electrons of the ligands enter into the vacant orbitals of the metal ion.Q2.
Initiation and propagation both involve bond homolytic cleavage (or fission, but cleavage sounds sexier). When only monosubstitution occurs (eg. excess alkane, limited halogen), then for 2,2-dimethylpropane, all hydrogens are equivalent (ie. gives the same product).Q3.
Option B generates a geminal diol, which readily dehydrates to form a ketone (or in this case, aldehyde).Q4.
Aminocarboxylic acid means both the amine group and the carboxylic acid group are present in the same molecule. In organic chemistry, the alpha carbon is desginated as the C atom directly bonded to the functional group. For amino acids of proteins, the highest priority goes to the carboxylic acid group, and the alpha C atom is the one bonded to the carboxylic acid group, and notice that (for amino acids of proteins) the amine group happens to be directly bonded to the alpha C atom as well, thus amino acids of proteins are called "alpha amino acids".
Any amino acid (whether alpha or beta or gamma, etc) are capable of forming zwitterions, because the amine group is basic and wants to accept a proton, and the carboxylic acid group is acidic and wants to donate a proton. When the amine group is protonated, the N atom gains a +ve formal charge. When the carboxylic acid group is deprotonated, the O atom gains a -ve formal charge. Thus forming a zwitterion. -
Hi,
I've encountered some questions and would like to discuss about some of their answers.
Q1: Which of the following statements about atomic orbitals are correct?
1....
2....
3. p orbitals are higher in energy than s orbitals.
The answer I have shows that statement 3 is wrong. Aren't p orbitals higher in energy than s orbitals?
Q2: Particle X has a proton number n and has a charge of +1. Particle Y has a proton number of (n+1) and is isoelectronic with X.
Which of the following is correct?
1. X is a neutral particle.
2. X is less electronegative than Y.
3. X and Y have similar shielding effect as they belong to the same period.
The answer is 2 & 3 are correct.
However, I was thinking that there is a possibility that 3 could be wrong (in the case of X being argon ion - Ar+ and Y being potassium ion K2+) Hence, they won't be belonging to the same period despite satisfying the conditions described above. Does what I think make sense?
Q3. The atom X has the electronic configuation is 1s2 2s2 2p6 3s2 3p3.
A.....
B: The element X forms the compound XCl3 with chlorine.
C: The 3s electrons of the element X are higher in energy than the 3p.
D....
The given answer is C - and I don't understand why. P does form the compound PCl3 with chlorine. Also, I thought 3s electrons are always lower in energy than the 3p? The answers in this question and Q1 do not reconcile with my understanding.
Q4:
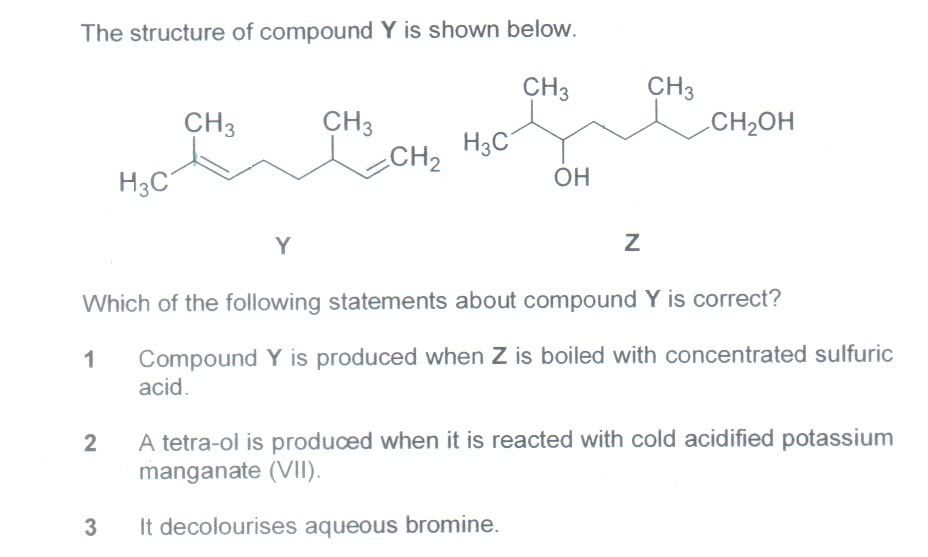
The answer is 2 & 3. Why is statement 1 wrong? Though I could have produced other compounds but compound Y is 1 of the compounds when Z is boiled with conc. sulphuric acid (dehydration) - am I right?
Any response would be much appreciated. :)
-
Originally posted by gohby:
Hi,
I've encountered some questions and would like to discuss about some of their answers.
Q1: Which of the following statements about atomic orbitals are correct?
1....
2....
3. p orbitals are higher in energy than s orbitals.
The answer I have shows that statement 3 is wrong. Aren't p orbitals higher in energy than s orbitals?
Q2: Particle X has a proton number n and has a charge of +1. Particle Y has a proton number of (n+1) and is isoelectronic with X.
Which of the following is correct?
1. X is a neutral particle.
2. X is less electronegative than Y.
3. X and Y have similar shielding effect as they belong to the same period.
The answer is 2 & 3 are correct.
However, I was thinking that there is a possibility that 3 could be wrong (in the case of X being argon ion - Ar+ and Y being potassium ion K2+) Hence, they won't be belonging to the same period despite satisfying the conditions described above. Does what I think make sense?
Q3. The atom X has the electronic configuation is 1s2 2s2 2p6 3s2 3p3.
A.....
B: The element X forms the compound XCl3 with chlorine.
C: The 3s electrons of the element X are higher in energy than the 3p.
D....
The given answer is C - and I don't understand why. P does form the compound PCl3 with chlorine. Also, I thought 3s electrons are always lower in energy than the 3p? The answers in this question and Q1 do not reconcile with my understanding.
Q4:

The answer is 2 & 3. Why is statement 1 wrong? Though I could have produced other compounds but compound Y is 1 of the compounds when Z is boiled with conc. sulphuric acid (dehydration) - am I right?
Any response would be much appreciated. :)
These are prelim paper qns, yes? Many prelim paper qns are of dubious quality, and I tell my students to take them (and their given answers) with a pinch of skepticism. Even Cambridge qns can sometimes include nonsense, let alone prelim paper qns. Don't give prelim qns too much credibility.Q1. Tis a trick qn. The qn setter is taking sadistic delight in that 2p orbitals are of lower energy than 3s orbitals (for instance).
Q2. Yes, you're correct. It could be either the atoms or ions, that have electron configuration corresponding to the same period. The qn does not clearly specify this.
Q3. Another unfair qn. The qn setter has in mind that since the 3s orbital is completely filled, inter-electron repulsion would cause the 3s electrons to have higher energy than the 3p orbitals, in which there is no intra-orbital electron repulsion (since they are all only half-filled). And regarding PCl3, in excess Cl2, PCl5 is more favourably generated. But you're right, this qn is unfair on both counts (the 3s vs 3p and the XCl3 options). Don't give prelim qns too much credibility.
Q4. You're right. Don't give prelim qns too much credibility.
-
Hi UltimaOnline, Many thanks for clarifying my doubts. :) These questions are actually Sec 4 IP EOY Questions. The setter didn't have much experience to set discriminating and accurate questions, I suppose. :P
-
I've another question here, don't quite understand the question.. Nitrogen (N), phosphorus (P), and potassium (K) are the main nutrients in plant fertilisers. According to an industry convention, the numbers on the label refer to the mass percents of N, P2O5, and K2O, in that order. Calculate the N:P:K ratio of a 30:10:10 fertiliser in terms of moles of each element, and express it as x:y:1.0. Thank you!
-
Originally posted by gohby:
I’ve another question here, don’t quite understand the question..
Nitrogen (N), phosphorus (P), and potassium (K) are the main nutrients in plant fertilisers. According to an industry convention, the numbers on the label refer to the mass percents of N, P2O5, and K2O, in that order. Calculate the N
 :K ratio of a 30:10:10 fertiliser in terms of moles of each element, and express it as x:y:1.0.
:K ratio of a 30:10:10 fertiliser in terms of moles of each element, and express it as x:y:1.0.Thank you!
Out of 100g, 30g are of N, 10g are of P2O5, and the last 10g are of K2O. Divide these sample masses by their molar masses to obtain number of moles, then give these values as a ratio of moles in the form x : y : 1.0 -
Originally posted by UltimaOnline:
Out of 100g, 30g are of N, 10g are of P2O5, and the last 10g are of K2O. Divide these sample masses by their molar masses to obtain number of moles, then give these values as a ratio of moles in the form x : y : 1.0Do you mean out of 50g instead? Since they asked for the ration in terms of the moles of each element, I will need to multiply P and K by 2 right?
-
Originally posted by gohby:
Do you mean out of 50g instead? Since they asked for the ration in terms of the moles of each element, I will need to multiply P and K by 2 right?
Yes, it's out of 50g (when I wrote 100g, I was thinking to write the masses as percentages, but changed my mind halfway and decided to leave them as masses, but neglected to amend the 100g).Yes, you need to multiply the moles of P2O5 and K2O by 2, to obtain the moles of P and K.