Chem Problem
-
Which one of the following is the number of electrons which have approximately the same
mass as that of a proton?A 20
B 200
C 2000
D 20000Choice C is the answer because the mass of an electron is 1/2000 of the mass off proton. Is this the reason??
-
Correct.
-
Both water and ice have H-bonding between their molecules. Ice is less denser than the liquid water because of the longer H-bonds in ice as compared to liquid water and secondly ice has expnaded structure.
What do you mean by the longer H-bonds in ice? Have they been meausred?
Open structure in ice...well the liquid water has also open structure. I did not get this. Please explain.
-
Originally posted by hoay:
Both water and ice have H-bonding between their molecules. Ice is less denser than the liquid water because of the longer H-bonds in ice as compared to liquid water and secondly ice has expnaded structure.
What do you mean by the longer H-bonds in ice? Have they been meausred?
Open structure in ice...well the liquid water has also open structure. I did not get this. Please explain.
The H bonds are 'forced' to be longer in ice, due to the rigid lattice structural arrangement in solid ice. No such rigid lattice arrangement occurs in liquid water, which means closer proximity, and therefore shorter (hence stronger) H bonds, are possible for liquid water.The difference in lengths of the H bonds in solid vs liquid water, is unlikely to be part of the Cambridge mark scheme (at least for the Singapore syllabus). The exact H bond length in solid ice, also depends on the exact cystalline form of ice, for which over a dozen different forms have been identified.
Interestingly, for Cambridge A level Chemistry, students need not be familiar with the most common crystalline form of ice, which is ice 1-hexagonal (because this would be more difficult for A level students), but instead Cambridge allows A level Chem students to draw out or describe the less common, but more familiar (due to the similarity to the lattice structure of diamond, which all 'O' and 'A' level Chem students are already familiar with) crystalline form of ice 1-cubic (ie. the diamond structure).
The so-called 'open structure' of ice, refers to the fact that there are lots of empty (or 'open') spaces between the H2O molecules in solid ice. In contrast, there is very little empty space between H2O molecules in liquid water.
Why? Because H2O molecules are forced to spread apart with some distance between them, in the fixed lattice (ie. regular repeating patterns) crystalline structures in solid ice (analogy : 100 disciplined soldiers doing a weapons precision drill parade, would have to keep some distance apart from each other and occupy a huge space in an open field), while in the liquid state, the H2O molecules are milling in close contact with each other, with no fixed lattice structure (analogy : 100 frenzied women now squeezing chaotically at some super discount sale in some small clothes/shoes departmental store).
This is the reason why solid ice is less dense than liquid water.
-
To get a more intuitive perspective on the phenomena, have a look at
http://www.youtube.com/watch?v=PcoiLAsUvqc
and http://www.youtube.com/watch?v=RIW65QLWsjE
These are simulations of water molecules transitioning from the liquid to solid phase.
-
How may nitrogen exist in compounds?
1 bonded by a triple covalent bond
2 as part of a cation
3 having lost 3 electrons to form an anion
All are correct.1 is true since N2 is present.
2 is true due NH4+
3 is also true due to NO2 -
Is it right Sir??
-
Yes.
Doh, I'm getting ahead of myself. I don't see N losing electrons for 3, so no.
-
Originally posted by hoay:
How may nitrogen exist in compounds?
1 bonded by a triple covalent bond
2 as part of a cation
3 having lost 3 electrons to form an anion
All are correct.1 is true since N2 is present.
2 is true due NH4+
3 is also true due to NO2 -
Is it right Sir??
The 3rd statement is ambiguous, and therefore this is a poorly set question.
There are three different ways in which chemists count electrons around an atom :
1) formal charge
2) stable octet
3) oxidation state
In the nitrate(III) anion, the N atom has 1 lone pair, and 3 bond pairs.
In terms of formal charge, this the N atom has 1x2 + 3x1 = 5 valence electrons, and because N is in Group V, hence its formal charge is 0.
In terms of stable octet, the N atom has 1x2 + 3x2 = 8 valence electrons, and thus indeed have a stable octet noble gas electron configuration.
In terms of oxidation state (OS), the N atom has lost 3 electrons to the more electronegative O atoms, and thus its OS = (formal charge + electronegativity consideration) = (0) + (+3) = +3.
-
.Actually this was the Q.31 of CIE MCQs paper 12 of Nov 2012. Marking schemes have not been given so far. I was just chcking my answers. So the answer for the above question is A?
Secondly there is another question no 33:
Which of these substances have a giant structure?
1 silicon(IV) oxide
2 baked clay found in crockery
3 phosphorus(V) oxideThe answer is B.
1 is correct.
3 is incorrect since it is simple molecular solid.
I have doubt about "baked clay"....But i remember that they crockery has high melting point so 2 is also correct....would you please explain baked clay.
-
Originally posted by hoay:
.Actually this was the Q.31 of CIE MCQs paper 12 of Nov 2012. Marking schemes have not been given so far. I was just chcking my answers. So the answer for the above question is A?
Secondly there is another question no 33:
Which of these substances have a giant structure?
1 silicon(IV) oxide
2 baked clay found in crockery
3 phosphorus(V) oxideThe answer is B.
1 is correct.
3 is incorrect since it is simple molecular solid.
I have doubt about "baked clay"....But i remember that they crockery has high melting point so 2 is also correct....would you please explain baked clay.
No shock there. You wouldn't believe how many poorly set, ambiguous questions came out in the recent years (2010, 2011, 2012) of the Singapore-Cambridge A Level H2 Chemistry papers. For such questions (for which there are multiple instances each year), school teachers disagree with each other and with Cambridge, on what the correct or best answer should be.
In such ambiguous questions, the exam-smart student has to cleverly deduce what Cambridge is probably thinking in setting the question. Probably, Cambridge intended to trap students by substituting "lost" for the more correct (and less ambiguous) statement of "the N atom can gain 3 electrons to form the nitride N3- anion", in which case the Cambridge mark scheme intended answer is probably that "statement 3 should be false".
For A level Chem purposes, "clay" or "ceramic" may be thought as mainly silicon oxide and aluminium oxide, although in actuality it has a highly variable composition of many compounds. In other words, a mixture of both a giant ionic lattice structure as well as a giant covalent lattice structure. Either way, option 2 is correct.
-
Many thanks for unceasing support and excellent explanation in the field of chemistry in general and A-level chemistry in particular.
Another question from nov 2012:
Q. Rat poison needs to be insoluble in rain water but soluble at the low pH of stomach contents. What is a suitable barium compound to use for rat poison?
A barium carbonate
B barium chloride
C barium hydroxide
D barium sulfateD is the answer.
And if i am not mistaken I used the salt hydrolysis approach for this question.
A will have ph > 7
B will have ph > 7
C will have ph > 7.
Is it correct?
-
Originally posted by hoay:
Many thanks for unceasing support and excellent explanation in the field of chemistry in general and A-level chemistry in particular.
Another question from nov 2012:
Q. Rat poison needs to be insoluble in rain water but soluble at the low pH of stomach contents. What is a suitable barium compound to use for rat poison?
A barium carbonate
B barium chloride
C barium hydroxide
D barium sulfateD is the answer.
And if i am not mistaken I used the salt hydrolysis approach for this question.
A will have ph > 7
B will have ph > 7
C will have ph > 7.
Is it correct?
Totally welcome.

The answer should be BaCO3.
Although both BaSO4 and BaCO3 are insoluble (ie. sparingly soluble) at neutral pH, in acidic pH only BaCO3(s) undergoes a reaction to generate the toxic Ba2+(aq) ions.
The solubility of BaSO4 remains largely unchanged with pH, since Ba(OH)2 and H2SO4 are both considered strong base and strong acid respectively, hence there is little common ion effect or shifting of positions of equilibrium.
The reaction of BaCO3(s) is as follows :
CO3 2- carbonate(IV) ions function as Bronsted-Lowry bases, and are protonated in acidic pH to generate H2CO3 carbonic(IV) acid, which exist in equilibrium with, and hence can decompose into, CO2(g) and H2O(l). Because CO2(g) leaves the reaction mixture, it pulls the position of equilibrium over to the right, as predicted by Le Chatelier's principle. Furthermore, non-gaseous reactants generating a gaseous product implies a positive entropy change of reaction, which is thus thermodynamically favourable and thermodynamicaly driven.
The toxic Ba2+(aq) is thus freed from the solid BaCO3(s) lattice structure, and available to do its merciless, murderous, lethal work coursing through the arteries and veins of the helpless rodent, who will soon experience multiple organ failure and die a terrible, excruciating, shrieking, convulsive, woeful death. The counter anion for the Ba2+(aq) ion, would be the Cl-(aq) ions of the HCl(aq) acid in the rat's stomach.
-
i thought electron mass is 1/1840 of proton?
-
Originally posted by a mugger:
i thought electron mass is 1/1840 of proton?
In MCQ, choose the 'best' answer, which is not necessarily the most accurate answer possible.Here, obviously 1 Sig Fig lor.
-
The answer in for Nitrogen question in the Marking scheme is B.
statement 3 is wrong. I think that the questions says existance of Nitrogen in compunds but 3 says that it is an anion may be NO2- which is an anion and not a compound, Is it the reason Sir?
Another thinking which is constantly bothering me....... O vs F
Oxygen in water or in any other molecule can make two H-bonds per molecule with itself the reason Two lone pairs on Oxygen. But flourine on the other hand only make only one H-bond per molecule despite the fact that f contains 3 lone pairs......Is it due to high electronegativitiy of F than O?? Or any other plausible explanation.
-
Originally posted by hoay:
The answer in for Nitrogen question in the Marking scheme is B.
statement 3 is wrong. I think that the questions says existance of Nitrogen in compunds but 3 says that it is an anion may be NO2- which is an anion and not a compound, Is it the reason Sir?
Another thinking which is constantly bothering me....... O vs F
Oxygen in water or in any other molecule can make two H-bonds per molecule with itself the reason Two lone pairs on Oxygen. But flourine on the other hand only make only one H-bond per molecule despite the fact that f contains 3 lone pairs......Is it due to high electronegativitiy of F than O?? Or any other plausible explanation.
For your first question, probably not. Afterall, not only would it be a silly question to challenge the A level student on semantics, but furthermore it can be argued that an anion is, together with some cation, still part of a compound.The more probable scenario is, Cambridge wanted the 'true' statement to be :
"An N atom can gain 3 electrons to form the nitride anion." True.
So they took out the word "gain" and replaced it with "lose" to form the 'false' statement :
"An N atom can lose 3 electrons to form the nitride anion." False.
------------------------------------------------------------------
In H-F molecules, because the F atom has 3 lone pairs, so in theory, it is possible for the F atom to be engaged in 3 hydrogen bonds simultaneously.
However, this does not happen for 2 reasons :
The 1st reason : each time a lone pair is used up to form a hydrogen bond, the other lone pairs (on the F, O, or N atom) are withdrawn away by induction (towards the hydrogen bond) and become less available to participate in it's own hydrogen bond.
Then why can the O atom of water use both of its lone pairs to participate in 2 hydrogen bonds simultaneously, as in solid ice? This is more possible for water than for hydrogen fluoride, because water has 2 H atoms that (being less electronegative) can donate its (bonding) electrons inductively toward the (more electronegative) O atom, resulting in the lone pairs of the O atom being more available to participate in their own hydrogen bonding. In contrast, hydrogen fluoride only has 1 H atom that donates inductively. With less 'income', the F atom is forced to be more 'miserly' with it's limited 'shopping money'.
The 2nd reason : each hydrogen fluoride molecule only has 1 partial positive H atom to participate in intermolecular hydrogen bonding, while in contrast each water molecule has 2. As an analogy : the classroom of water has 2 guys (lone pairs) and 2 girls (partial positive H atoms); the classroom of ammonia has 1 guy (lone pair) and 3 girls (partial positive H atoms); the classroom of hydrogen fluoride has 3 guys (lone pairs) and 1 girl (partial positive H atom).
How many heterosexual (non-gay, non-lesbo) relationships can each class have? Obviously, water will have the most number of intermolecular hydrogen bond relationships, and therefore water has a higher boiling point than either ammonia or hydrogen fluoride.
While water as solid ice can gain maximum thermodynamic stability by forming 4 hydrogen bonds per water molecule in a tetrahedral geometry (eg. in ice 1-cubic); in contrast for hydrogen fluoride, the maximum thermodynamic stability (ie. most efficient way of arranging themselves to achieve maximum number of hydrogen bonds for the entire population) is achieved by forming zig-zag chains of HF in the liquid and solid states, with each HF molecule having 2 primary (semi)-permanent hydrogen bonds within each chain (as shown below); and between the chains, secondary (and weaker, more temporary) hydrogen bonds and/or permanent dipole - permanent dipole Keesom van der Waals interactions may occur.
-
Excellent explanation.
you said above "While water as solid ice can gain maximum thermodynamic stability by forming 4 hydrogen bonds per water molecule in a tetrahedral geometry"..... This is not totally correct. in ice two bonds are covalent while two bonds are H-bonds making a total of four.
The following question also appered in A-level Nov 2012 CIE variant 13. the above N-question appeared in the same session but variant was 12.
How may nitrogen exist in compounds?
1 bonded by a triple covalent bond
2 as part of a cation
3 in an oxidation state of +5The answer according to CIE MS is A.
1 is correct due to N2
2 is correct due to NH4+
3 is also correct due to what I am thinking is due to Nitrogen in HNO3.
-
Originally posted by hoay:
Excellent explanation.
you said above "While water as solid ice can gain maximum thermodynamic stability by forming 4 hydrogen bonds per water molecule in a tetrahedral geometry"..... This is not totally correct. in ice two bonds are covalent while two bonds are H-bonds making a total of four.
The following question also appered in A-level Nov 2012 CIE variant 13. the above N-question appeared in the same session but variant was 12.
How may nitrogen exist in compounds?
1 bonded by a triple covalent bond
2 as part of a cation
3 in an oxidation state of +5The answer according to CIE MS is A.
1 is correct due to N2
2 is correct due to NH4+
3 is also correct due to what I am thinking is due to Nitrogen in HNO3.
My original statement regarding hydrogen bonds in ice remains correct.
Each water molecule can have up to four simultaneous hydrogen bonds in ice. Different crystalline forms of ice will have different number of hydrogen bonds per water molecule.
For instance, in one crystalline form of ice, each water molecule has three intermolecular hydrogen bonds, as shown below :
In another crystalline form of ice, each water molecule has four intermolecular hydrogen bonds (as shown) :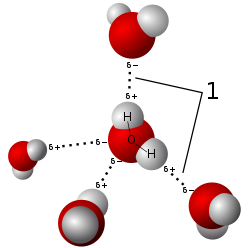
In practice, similar to amorphous carbon, in which there is a mixture of the different allotropes of carbon present, most samples of solid ice consists of a mixture of the various crystalline forms of ice, in which some water molecules have 2 intermolecular hydrogen bonds, while some have 3 intermolecular hydrogen bonds, and some other water molecules have up to 4 intermolecular hydrogen bonds, as shown below :

For A level purposes, as long as the diagram is labelled correctly, full credit will be awarded. The following 4 points may be used as a checklist in the Cambridge Mark Schemes.
1) All relevant partial charges must be shown.
2) Length of hydrogen bonds must be slightly longer than covalent bonds.
3) The lone pair on the hydrogen bond acceptor must be shown at the base of the hydrogen bond.
4) The geometrical angle of the hydrogen bond to the H atom to the covalent bond, must be linear (ie. 180 deg).(Note : many Singapore JCs don't teach point #4, and admittedly it is not always possible to show point#4 in 2D-drawings, but there have been Cambridge Mark schemes which require this marking point, so the exam-smart student will play safe and show point#4 whenever possible.)
-----------------------------------------------------------
Regarding your Nitrogen question :
Correct, the OS of N atom in HNO3 (conjugate acid) and NO3- (conjugate base), is +5.
Oxidation State (OS) = Formal Charge + Electronegativity Consideration.
Apply this formula to any 1 of the 3 resonance contributors here :

Oxidation State (OS) of the N atom
= Formal Charge + Electronegativity Consideration
= (+1) + (+1 +2 +1)
= +5Whether you apply the formula to the resonance contributors or the resonance hybrid, or to the conjugate acid or the conjugate base, the OS of the N atom remains as +5.
In some other molecules, eg. the dinegative tetrathionate anion, applying my BedokFunland JC formula (ie. a University level formula, that most Singapore JCs teach only to their H3 and Olympiad students, leaving the pitiful H2 students in the cold) for Oxidation State (OS) yields :

For the oxo-bonded S atoms :
Oxidation State (OS) = Formal Charge + Electronegativity Consideration = (0) + (+5) = +5
and for the thio-bonded S atoms :
Oxidation State (OS) = Formal Charge + Electronegativity Consideration = (0) + (0) = 0
Hence the average OS of sulfur in the thiosulfate anion = [ (+5) + (0) + (0) + (+5) ] / 4 = +2.5 -
The formation of covalent bond in terms of orbital overlap for HCl describes the overlap between a 2px and 1s orbitals of Cl and H resulting in the formation of sigma bond.
The valence-shell electronic configuration of Cl is 3s2, 3px2,3py2,3pz1 which means that it is the 2pz-orbital which should overlap with the 1s -orbtals. But the overlap between the pz and s is not possible.
Please explain these two things that (i) why px is chosen to overlap with 1s and not pz.
(ii) why the overlap between the pz and s is not possible.
-
Originally posted by hoay:
The formation of covalent bond in terms of orbital overlap for HCl describes the overlap between a 2px and 1s orbitals of Cl and H resulting in the formation of sigma bond.
The valence-shell electronic configuration of Cl is 3s2, 3px2,3py2,3pz1 which means that it is the 2pz-orbital which should overlap with the 1s -orbtals. But the overlap between the pz and s is not possible.
Please explain these two things that (i) why px is chosen to overlap with 1s and not pz.
(ii) why the overlap between the pz and s is not possible.
This is a non-issue for A levels. For A level purposes, tell your students to just write, "the sigma bond between H and Cl in the HCl molecule, is formed by the head-on or end-on overlap between the 1s orbital of H atom, and a 3p (if assumed to be unhybridized) or a 3sp3 (if assumed to be hybridized) orbital of Cl."Which 3p orbital exactly? It gets a little complicated here (and totally not required by A levels).
If you consider that Cl hybridizes its orbitals accordingly to VSEPR theory, than because there are 3 lone pairs + 1 bond pair = 4 electron charge clouds, then the Cl orbital involved in forming the sigma bond would be a 3sp3 hybridized orbital.
If you consider the fact that Cl being in period 3 has a large atomic radius, whose electron pairs are too far away from each other, and experience little repulsion with each other, then the Cl atom wouldn't need to expend energy to hybridize its orbitals, and therefore an unhybridized orbital would be used to form the sigma bond.
Since the 3pz orbital is singly occupied, then that would be the orbital utilized. However, px, py and pz orbitals are merely human labels, in actuality these are arbitrary as the p orbitals are degenerate, with any one being singly occupied and the other two being doubly occupied (the atom itself has no recognition of x, y or z, which are human terms).
To complicate matters further, a more 'correct' model used to describe the nature of molecular bonds, would be the Molecular Orbital theory, which is a concept too far beyond the A levels to be of any value whatsoever to the A level student (whether it be the Singapore H1/H2/H3 student, or the CIE student).
So in summary, this question is completely a non-issue for the A level student, and should not be of any concern whatsoever to you, or to your students.
If you're asking beyond A levels, then that is not my area of professional interest. You can read up on Molecular Orbital theory on Wikipedia, and/or ask on TheChemicalForums (all questions beyond A levels should be posted therein).
-
4) The geometrical angle of the hydrogen bond to the H atom to the covalent bond, must be linear (ie. 180 deg).
(This I think is covered in H3 ....)
-
Bio I mean
