2017 H2 Chemistry JC1 & 2 students post your questions here
-
Originally posted by jyjyjy:
JJC P2 Q1(e) why is it that even though reaction is independant of [I2], when [I2] is halved, time is halved? wouldn't that be first order?
Q5(c)(iii) How does this mechanism look like? Is it possible for the Br- from step 1 to attack instead?
P3 Q1(b)(ii) Why does the answer state that when the reaction just becomes feasible G=0? Isn't the reaction feasible when G<0? When G=0 isn't it just at equilibrium?
P2 Q1e) If I2 was 1st order, then the half-life would be constant, ie. no change. Since I2 is a 0th order reactant, half-life is halved when molarity is halved.P2 Q5ciii) SN1. If the leaving group Br- attacked back, then you would end up with the original reactant, ie. no net reaction occurred.
P3 Q1(b)(ii) Yes you're right, but what exact value are you going to substitute in for delta G? -1? -0.1? -0.01? -0.001? So just use delta G = 0 first, then state that for the reaction to be thermodynamically feasible, the temperature needs to be higher than 958K (ie. take initiative to improve on the given mark scheme answer, when answering such questions in the A levels).
-
^_^
-
Just wanna enquire usually for P2 what is the average % range for students who are generally good at chem?
Based on my past tys P2 practices my % score is hovering around 85% or so.78-82% if that particular year theres a transition metal question(provided it is a rather difficult question,otherwise its easy).
-
Originally posted by MapPwner:
Just wanna enquire usually for P2 what is the average % range for students who are generally good at chem?
Based on my past tys P2 practices my % score is hovering around 85% or so.78-82% if that particular year theres a transition metal question(provided it is a rather difficult question,otherwise its easy).
You're on track to get A grade, no worries. -
^_^
-
^_^
-
^_^
-
A BedokFunland JC H2 Chemistry Qn : Why do soft drinks foam up a lot more when poured out of a can on an aircraft during a flight?
Btw, Lee Hsien Loong's effort to combat diabetes in Singaporeans notwithstanding, you should still always go for Regular Coca-Cola over either Coca-Cola Zero or Coca-Cola Light (marketed as Diet Coke in some countries), because artificial sweeteners are always even more unhealthy than natural sugar.
https://sg.yahoo.com/news/one-drink-never-order-plane-185748754.html
-
[Medicine] - A significant % of the human population suffers from defective MTHFR genes, which results in elevated Homocysteine levels, which predicts (ie. may or may not cause, medical evidence only supports correlation, not causation) significantly increased risks of cardiovascular disease and strokes. MTHFR gene mutations also cause significantly decreased Glutathione levels, and is also strongly linked to at least 60 different medical conditions.
Dr Amy Myers : What is an MTHFR Mutation and What Can We Do About It?
http://www.amymyersmd.com/2017/07/what-is-an-mthfr-mutation-and-what-to-do-about-it/
Homocysteine and MTHFR Mutations : relation to Thrombosis and Coronary Artery Disease
http://circ.ahajournals.org/content/111/19/e289Homocysteine : Associated Diseases, Link to Vitamin Bs, Homocysteine Reduction Methodologies
http://www.lifeextension.com/Protocols/Heart-Circulatory/Homocysteine-Reduction/Page-02Methylation and Homocysteine : Factors and Treatment
http://www.foodforthebrain.org/alzheimers-prevention/methylation-and-homocysteine.aspx--------------------------------------------------------------------------------
Damned if you do, damned if you don't. Welcome to physical incarnation.
Migraine sufferers have defective MTHFR genes and require High Vitamin Bs intake to reduce Homocysteine levels to prevent Migraines
http://articles.mercola.com/sites/articles/archive/2009/04/21/b-vitamins-offer-migraine-relief.aspxMedical Study finds Link between High Vitamin Bs intake and Lung Cancer in men
https://sg.yahoo.com/style/study-finds-between-high-vitamin-b-intake-lung-123029252.html--------------------------------------------------------------------------------
L-Methylfolate (5-MTHF) supplementation required for people who cannot process folic acid due to MTHFR gene mutation
https://www.dietvsdisease.org/l-methylfolate-5-mthf/
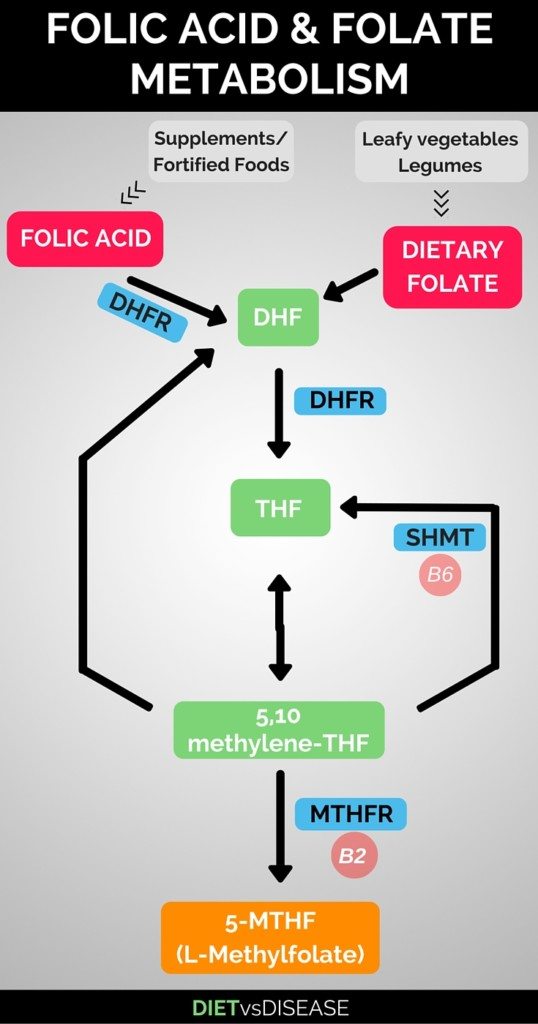
--------------------------------------------------------------------------------
https://en.wikipedia.org/wiki/Levomefolic_acid--------------------------------------------------------------------------------
Dr Lynch explains why (6S)-5-methyltetrahydrofolic acid is the correct enantiomer (H2 Chemistry) that is required as supplementation for sufferers of MTHFR gene mutations :
http://mthfr.net/l-methylfolate-methylfolate-5-mthf/2012/04/05/[Pharmaceutical Chemistry] - Different Types of Methylfolate, including Metafolin® versus Quatrefolic®
http://www.podiapn.com/
http://methyl-life.com/methylfolate-types/--------------------------------------------------------------------------------
Question : I was wondering about the connection between MTHFR and glutathione you mentioned in your video presentation. It seems to me that if there is a MTHFR defect, resulting in undermethylation, that should result in increased availability of homocysteine to be converted to cysteine, and ultimately to formation of glutathione. Yet you are saying there may be problems with glutathione production in MTHFR mutations. Can you explain why? - A fellow medical doctor.
Dr Lynch replies : Great question. Short term, you are correct. Short term, the increase in CBS enzyme activity should lead – and does lead – to increased glutathione production. The problem is, long term, with MTHFR, and oxidative stress, the glutathione oxidation increases beyond the point of glutathione production. This is because the traditional transmethylation cycle (Methionine cycle) and BHMT pathway help produce CoQ10, carnitine, phosphatidylcholine, creatine, SAMe – all of which are big players in antioxidant production and mitochondrial function. As those decline, oxidative stress increases, CBS upregulation is even higher – and in turn, due to decreased CoQ10, carnitine, creatine, etc, ammonia levels climb and potentially so does hydrogen sulfide levels – and the inability for the sulfonation pathway to keep up with the increased sulfite/sulfide production thus leading to sulfur sensitivity and molybdenum deficiency. I should also add that the likelihood of cysteine, glycine and B6 levels declining are high due to long term oxidative stress. Then – the production of glutathione is also affected. Not to mention the decline of vitamin C, selenium and vitamin E which help prevent oxidized glutathione and help recycle it back into reduced glutathione. I believe that if we support the levels of CoQ10, carnitine, creatine, magnesium, phospholipids, molybdenum, decreased sulfur foods initially – and decreased sulfur supplements – and possibly increase SAMe right out of the gate – before even supplementing with glutathione, methylfolate or methylcobalamin, the degree of improvement should increase quickly. As improvement occurs, then supporting phase 2 with glutathione, NAC and glycine should further help. I think now if we support the MTHFR defects this way – by reducing oxidative stress, improving cell membrane stability and replenishing mitochondrial and antioxidant levels – our patients will improve much faster. Then – once those are in play – then supporting MTHFR for long term natural production and hopefully removing that initial supplementation. - Dr Lynch - https://seekinghealth.org/resource/podcast-8-mthfr-and-glutathione-production/
See Dr Lynch's "Supplements to Reduce Homocysteine Levels"
http://mthfr.net/comparison-of-homocysteine-support-products/2011/09/13/--------------------------------------------------------------------------------
MTHFR gene, Homocysteine and Vitamin B12 : A comparison of different approaches by Dr. Ben Lynch versus Dr. Amy Yasko versus Dr. James Braly
http://mthfrliving.com/health-tips/supplementing-for-mthfr-b12/
-
Vitamin B2 Riboflavin is one of the touted remedies for Migraines, but be careful with it.
https://migraine.com/migraine-treatment/natural-remedies/riboflavin-vitamin-b2/
The 400mg dose used in the medical studies for treating migraines is extremely high, and will cause multiple organ toxicity if you're exposed to sunlight (ie. specifically UV light, also generated by some indoor lighting), as well as upset the balance between the other B vitamins.
Vitamin B2 Riboflavin Can Be Toxic with Sunlight Exposure
http://www.smart-publications.com/articles/excess-vitamin-B2-riboflavin-can-be-toxic"I concur that excessive B2 as well as B1 and B3 can make the need for methylfolate B9 and potassium become insatiable and send Serotonin levels plunging and many other problems. The balance with the other B vitamins has to be found by titrations and varies depending on your genetics." - http://forums.phoenixrising.me/index.php?threads/no-love-of-b2-here-a-warning-about-riboflavin.31639/
On a related medical biochemistry note, in the same discussion thread :
"Why excessive vitamin B2 causes a reduced amount of Serotonin : B2 is used in 3 very important enzyme complexes that are related to energy production, and that also use B1 Thiamine : the pyruvate dehydrogenase complex, the alpha-ketoglutarate dehydrogenase complex, and the branched chain ketoacid dehydrogenase complex (which allows valine, etc, to enter the TCA cycle as intermediates or acetyl CoA). If a person is low on B1 Thiamine, then taking B2 without B1 Thiamine (and you need more of the B1 Thiamine than the B2), they could drive their B1 Thiamine levels even lower because taking B2 will push these enzymes, which also use B1 Thiamine. B1 Thiamine is also used in the Pentose Phosphate pathway, or PPP, which provides NADPH for Glutathione reductase, among many other pathways. With low B1 Thiamine, the NADPH production from the PPP is reduced and then the body has to make up the difference through the Folate cycle. It isn't well known that the Folate cycle can produce NADPH, but it is. When glycine is used to make 5,10-methylene THF, ammonia is released. What do you need to get rid of ammonia? BH4. What is one of the things you need to make Serotonin? BH4. What happens is that the other amino acids are shunted into making serine and glycine for NADPH synthesis and so the urine amino acids are low, except for taurine, which can't enter the TCA cycle. The amino acids enter the TCA cycle and when they become malate they can enter the pathway to make serine and glycine. This is what showed up on my husband's urine amino acids test, with all the essential amino acids very low except taurine. My husband has had minor depression for years, and also high ammonia for years (we can smell it in his sweat and it comes out on urine tests.) It doesn't matter whether he has a high protein or low protein diet, his sweat smells, or smelled, like ammonia. We felt that it might cause a problem with his BH4, but we couldn't see what to do about it. Well, 3 days ago he started taking 1000mg of thiamine, instead of 100mg, along with his other vitamins, and last night he came in after exercising and sweating like crazy, and he said, "My shirt doesn't smell like ammonia now when I exercise!" And it didn't!"
-
TriMethylGlycine (TMG) can be very helpful with reducing harmful Homocysteine levels, especially for those with MTHFR gene mutations. However, as the following cases illustrate, be careful with TMG supplementation.
A woman (wiltedflower77) with the following genotypes sought advice regarding her nasty experience with TMG supplementation :
MAO rs6323 +/+
MTHFR c677T +/-
MTRR A66G +/-
CBS A360A +/-
VDR Bsm +/+
SOD2 A16V +/-"I tried taking about 200mg TMG powder in the morning. By next day I am severely depressed, suicidal, very tearful, and angry. The next day after stopping it I feel better mentally. I just don't understand what's happening???" - http://forums.phoenixrising.me/index.php?threads/tmg-makes-me-very-depressed-why.45321/
In the same discussion thread, a man (Hip) referenced a post he made previously :
"I have worked out the underlying biochemical mechanism why TMG, SAM-e and betaine hydrochloride cause me to feel depressed : http://forums.phoenixrising.me/index.php?threads/just-got-my-methylation-and-detox-profile-results-can-the-experts-kindly-provide-commentary-please.22304/page-2#post-351180"
-
PJC P3 Q4(a) How did they manage to come to the conclusion that G contains basic/amine group and phenol from G reacting w NaOH and HCL? Also how to conclude that K has 2 phenol groups from K not reacting with PCl5?
5(a)(i)Why did the answer key show -1.02V in one line and +1.02V in the next?
P2 Q6 (b) what does it mean by ring/angle strain?
-
Originally posted by jyjyjy:
PJC P3 Q4(a) How did they manage to come to the conclusion that G contains basic/amine group and phenol from G reacting w NaOH and HCL? Also how to conclude that K has 2 phenol groups from K not reacting with PCl5?
5(a)(i)Why did the answer key show -1.02V in one line and +1.02V in the next?
P2 Q6 (b) what does it mean by ring/angle strain?
PJC P3 Q4(a). Ask yourself to compare the solubility of amines before and after adding HCl, and the solubility of phenols before and after adding NaOH, and you have your answer.K comes from G, which contains 3 OH groups (since there is no COOH group since G doesn't react with Na2CO3, and the idea of the O atoms being carbonyl C=O groups is inconsistent in terms of degree of unsaturation with the subsequent reaction products implying presence of a benzene ring), 1 of which must be a secondary alcohol (since J reacts with 2,4-DNPH but not with Tollens) which is eliminated during dehydration to form K, hence the 2 remaining OH groups, which don't react with PCl5, must be phenolic OH groups, which is consistent with K being oxidized by KMnO4 to L (use degree of unsaturation to deduce benzene ring oxidation).
Q5ai) The -1.02V refers to standard oxidation potential of tartaric acid to CO2, but the question asks you for standard reduction potential of CO2 to tartaric acid, that's why.
P2Q6b) 3 or 4 membered lactone rings are strained (ie. ring strain), because their angles deviate from the ideal bond angles as predicted by VSEPR theory (ie. angle strain).
-
Sat for my school's prelim practical exam today for H2 Chemistry,55 marks in 2hrs30mins.Was a total disaster.Wrong manipulation of results and erroneous readings often,ended up with an overly concentrated NaOH soln to be determined between 1.5-2moldm3(turned out all my frens results were 2.3-2.5moldm^-3 so lab technician probably overdid it).
Best part was anxiety and panic got the better of me for this question below:
Using certain reagents,distinguish between the cations determined in part(i),which are Mg2+ and Zn2+,to determine which cation it actually is.(4 Marks).
Used NaOH dropwise,white ppt formed.Excess still ppt present at bottom and i thought it was Mg2+.Used NH3 dropwise,same ppt but in excess all dissolved.I ended up yolo-whacking Mg2+(shld have put zn2+ instead).Well at least i wrote the observations one should obtain if they get Mg2+ as the answer even though i wrongly answered the question in determining the correct cation,so i assume 2/4 marks.Most people skipped this question.
-
Originally posted by UltimaOnline:
You're on track to get A grade, no worries.After my chemistry prac prelim today,i am doubting my capabilities now haha.But if even the Band 1 students in my school chemistry cohort can't finish and made severe careless mistakes,then I'm not that worried,for now.
-
Originally posted by MapPwner:
Sat for my school's prelim practical exam today for H2 Chemistry,55 marks in 2hrs30mins.Was a total disaster.Wrong manipulation of results and erroneous readings often,ended up with an overly concentrated NaOH soln to be determined between 1.5-2moldm3(turned out all my frens results were 2.3-2.5moldm^-3 so lab technician probably overdid it).
Best part was anxiety and panic got the better of me for this question below:
Using certain reagents,distinguish between the cations determined in part(i),which are Mg2+ and Zn2+,to determine which cation it actually is.(4 Marks).
Used NaOH dropwise,white ppt formed.Excess still ppt present at bottom and i thought it was Mg2+.Used NH3 dropwise,same ppt but in excess all dissolved.I ended up yolo-whacking Mg2+(shld have put zn2+ instead).Well at least i wrote the observations one should obtain if they get Mg2+ as the answer even though i wrongly answered the question in determining the correct cation,so i assume 2/4 marks.Most people skipped this question.
Yeah Practical's like that, everyone also kena such problems, no worries. Focus on what you can control, ie. scoring near full-marks for the Theory papers, and just do your natural, stress-free best for the Practical. I every year lepak-lepak throw away both my Practical paper and my Planning Qn (ok from this year on, they're both in the same Paper), and I still get my A grade every year, stress-free. Be like me lor ;Þ -
Ok, this isn't a Health Hack per se (although some people have used the sleepy effect as a hack to help overcome insomnia), but more of an FYI informative post.
Did you know... raw onions, raw garlic and durians have peculiar effects on some people, such as making you thirsty and/or sleepy? Such 'allergic' reactions are genetically determined (by a number of inter-related genes), and are certainly associated with the various metabolic biochemical cycles and systems that we've been looking at, in the other posts here.
https://www.google.com.sg/search?source=hp&q=onions+thirsty
https://www.google.com.sg/search?source=hp&q=onions+sleepy
https://www.google.com.sg/search?source=hp&q=garlic+thirsty
https://www.google.com.sg/search?source=hp&q=garlic+sleepy
Need a clue? What do all 3 (onions, garlic and durians) have in common? Their pungency? Which is caused by? That's right, sulfur-rich compounds (eg. proteins rich in cysteine amino acid).
As you should know by now (reading my other posts in my Health Hacks forum), Glutathione is a key anti-oxidant which requires the sulfur-containing cysteine amino acid (which is why N-AcetylCysteine is often used by medical doctors to replenish a patient's Glutathione levels).
And the metabolic biochemical cycles and systems involved in generating and regulating Glutathione levels, also involve the MTHFR gene, all the B Vitamins, as well as the undermethylation versus overmethylation issue.
It's therefore not surprising, that sulfur-rich foods such as onions, garlic and durians, have such peculiar effects on some people. And what exactly it means for people 'allergic' to these foods, in terms of the MTHFR and associated genes, the undermethylation versus overmethylation issue, is not so straightforward and little understood even by medical doctors at the current time.
-
North Korea has upgraded it's nuclear weapons arsenal : it just successfully detonated a hydrogen nuclear bomb, where previously it only had used atomic nuclear bombs.

https://sg.yahoo.com/news/north-korea-explosion-points-nuclear-test-043842707.html
[Chemistry / Physics] - A-bombs vs. H-bombs: What's the difference?
-
^_^
-
This should have been intuitive and not surprising : any chemical that uses its powerful biologically destructive effect, ie. most bleaches employ strong oxidizing agents such as sodium hypochlorite aka sodium chlorate(I) for its bleaching, cleaning, disinfecting functions, will most certainly have similarly destructive effects on the human biological body.
Many restaurants in Singapore use bleach to wipe tables and mop floors even when customers (eg. myself) are present and still eating, the bleach smell has always nauseated me and I've always intuitively known there would be harmful damaging biological effects, even on customers with occasional exposure, let alone clueless, hapless and helpless employees using the bleach (similar to some pitiful employees working under installed UV lightings at their workplace, eg. Singapore Zoo / Night Safari, without realizing the carcinogenic DNA damage UV light causes, which is also why you should get your anti-cancer Vitamin D from supplements rather than from sunlight exposure, no thanks to ozone layer depletion).
Regularly using bleach linked to higher risk of fatal lung diseases such as COPD
https://www.google.com.sg/search?source=hp&q=bleach+lung+disease
-
An Original H2 Chemistry BedokFunland JC Quick-Riddle :
Related to the previous post above, sodium hypochlorite [Latin name] or sodium chlorate(I) [Stock name] is often used as the active oxidizing ingredient in most Bleach solutions (this varies with brand). NaClO is obviously an ionic compound, which is thus non-volatile. Yet, you can certainly smell Bleach, and the vapors have now been shown in medical studies to cause irreversible oxidative damage to your lung alveoli tissue, resulting in severe lung diseases such as COPD. Explain this apparent contradiction.
-
Hello! May I ask if there is a need to explain why permanent dipole-permanent dipole attractions are stronger than instantaneous dipole-induced dipole attractions when answering a qn asking for the reason in difference in melting/boiling point of different molecules? If yes, what should I include?
-
Originally posted by Claresse216:
Hello! May I ask if there is a need to explain why permanent dipole-permanent dipole attractions are stronger than instantaneous dipole-induced dipole attractions when answering a qn asking for the reason in difference in melting/boiling point of different molecules? If yes, what should I include?
Yo!No need to explain further.
If one species has intermolecular hydrogen bonding while the other only has intermolecular van der Waals interactions, there is no need to specify the type of van der Waals interactions.
If both species only have intermolecular van der Waals interactions, then the most important point to consider (ie. which outweighs the polarity factor described next) is the size of the molecule and total number of electrons in the species.
Only if both species have similar molecular size and total number of electrons, then you consider the polarity of the 2 species, and bring in the type of van der Waals (ie. only for similar molecular size and number of electrons, then Keesom van der Waals forces outweigh Debye van der Waals forces outweigh London dispersion van der Waals forces).
Another factor to consider (for organic chemistry alkanes, etc), is branching, which reduces the surface area available for van der Waals interactions.
And for alkenes, the cis isomer (having a greater polarity) has a higher boiling point than the trans isomer, but the cis isomer may (or may not) have a lower melting point than the trans isomer, because there is the additional factor of effectiveness of stacking, which relates to the Coloumb's law and strength of van der Waals interactions.
-
-
Hi, can I ask if you have any really challenging organic chemistry structure elucidation questions to practice?
