2017 H2 Chemistry JC1 & 2 students post your questions here
-
Originally posted by CB2883J:
Btw what is meant by stereoisomer here? The kind of isomerism I’m getting is positional/constitutional/structural isomerism.
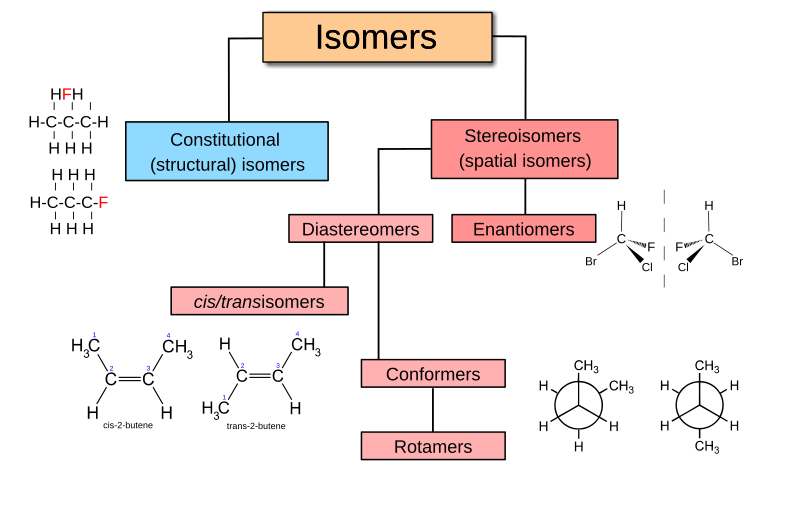
-
Originally posted by UltimaOnline:
Right, suspected my answer was wrong as soon as I posted. Thanks for clarifying. Though - does a pair of enantiomers count as two stereoisomers? (I'm having a hard time seeing how a third stereoisomer of the same compound can be achiral)
-
Originally posted by CB2883J:
Right, suspected my answer was wrong as soon as I posted. Thanks for clarifying. Though - does a pair of enantiomers count as two stereoisomers? (I'm having a hard time seeing how a third stereoisomer of the same compound can be achiral)
Of course, as you said, it's a "pair", ie. 2.Hint : it straddles both the H2 and H3 syllabuses, but came out for the Singapore H2 A Level Exams in 2012.
-
Originally posted by UltimaOnline:
Unfortunately, both CB2883J and delurach gave the wrong answers, because both of you offered constitutional isomers, instead of stereoisomers of the same alkane.Now that you guys mention it, I've another related BedokFunland JC Challenge Qn that allows for constitutional isomers, you guys can give this a try.
How many monochlorinated isomers (including both constitutional isomers and stereoisomers) can be generated when 3-methylpentane is reacted with Cl2 under UV light? Name all of them.
1-chloro,3-methylpentane
2-chloro,3-methylpentane
3-chloromethylpentane
3-chloro,3-methylpentane
1-chloro,2-ethylbutane
2-chloro,2-ethylbutane
2-chloroethylbutane
3-chloro,2-ethylbutane
1-chloro,3-ethylbutane
1-chlorohexane
2-chlorohexane
3-chlorohexane
1-chloro,2-methylpentane
2-chloro,2-methylpentane
2-chloromethylpentane
3-chloro,2-methylbutane
1-chloro,4-methylbutane
2-chloro,4-methylbutane
3-chloro,4methylbutane
ok i don’t know where this is going...
-
On a related note, here are a couple more BedokFunland JC riddles for your amusement :
Q1. This question illustrates why E-Z naming system is superior to the cis-trans naming system. If you insisted on forcibly naming (like how Singapore JCs do) the following molecule using the inferior cis-trans system, how would you name it?
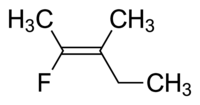
Q2. How many isomers, including both constitutional and stereoisomers, exist for C3H6O?
-
Originally posted by delurach:
1-chloro,3-methylpentane
2-chloro,3-methylpentane
3-chloromethylpentane
3-chloro,3-methylpentane
1-chloro,2-ethylbutane
2-chloro,2-ethylbutane
2-chloroethylbutane
3-chloro,2-ethylbutane
1-chloro,3-ethylbutane
1-chlorohexane
2-chlorohexane
3-chlorohexane
1-chloro,2-methylpentane
2-chloro,2-methylpentane
2-chloromethylpentane
3-chloro,2-methylbutane
1-chloro,4-methylbutane
2-chloro,4-methylbutane
3-chloro,4methylbutane
ok i don’t know where this is going...
Wrong. You're not allowed to use chain isomers, the question specified "3-methylpentane". -
Originally posted by UltimaOnline:
Of course, as you said, it's a "pair", ie. 2.Hint : it straddles both the H2 and H3 syllabuses, but came out for the Singapore H2 A Level Exams in 2012.
Is it a c-c bond with an internal plane of symmetry? the question of lactic acid whereby it polarises right and left?
-
Originally posted by UltimaOnline:
Of course, as you said, it's a "pair", ie. 2.Hint : it straddles both the H2 and H3 syllabuses, but came out for the Singapore H2 A Level Exams in 2012.
Oh, is it 3,4-dimethyl hexane with chiral chiral nonchiral (the last one with the internal plane of symmetry) (should be what delurach said)
-
Originally posted by delurach:
Is it a c-c bond with an internal plane of symmetry? the question of lactic acid whereby it polarises right and left?
*Nods*. Carry on.(You still need to draw out the 3 stereoisomers, and give a proper textual explanation, for full marks, if Cambridge asks this qn tmrw).
-
Two chiral centres hence theoretical max 4 isomers but if which two are the same (which is the internal plane of symmetry one)
-
Originally posted by CB2883J:
Oh, is it 3,4-dimethyl hexane with chiral chiral nonchiral (the last one with the internal plane of symmetry) (should be what delurach said)
*Nods*. Carry on.(You still need to draw out the 3 stereoisomers, and give a proper textual explanation, for full marks, if Cambridge asks this qn tmrw).
-
Originally posted by CB2883J:
Two chiral centres
*Nods*. Carry on.(You still need to draw out the 3 stereoisomers, and give a proper textual explanation, for full marks, if Cambridge asks this qn tmrw).
-
Originally posted by UltimaOnline:
*Nods*. Carry on.(You still need to draw out the 3 stereoisomers, and give a proper textual explanation, for full marks, if Cambridge asks this qn tmrw).
https://i.imgur.com/Pjkv9gu.jpg
Pardon the messiness
-
Originally posted by delurach:
https://i.imgur.com/Pjkv9gu.jpg
Pardon the messiness
Good, you drew out the meso isomer correctly.But you need to draw the pair of enantiomers side-by-side as mirror images of each other, otherwise you won't get the mark.
And of course, none of you gave the required textual explanation yet.
-
Originally posted by UltimaOnline:
Good, you drew out the meso isomer correctly.But you need to draw the pair of enantiomers side-by-side as mirror images of each other, otherwise you won't get the mark.
And of course, none of you gave the required textual explanation yet.
Is it just
1) there is an internal plane of symmetry and hence does not rotate plane polarised light, therefore is superimposable and hence is not a meso compound
2) for the other 2, they are both optically active and non-superimposable and hence exhibits optical isomerism and hence are stereoisomers
-
Originally posted by CB2883J:
Two chiral centres hence theoretical max 4 isomers but if which two are the same (which is the internal plane of symmetry one)
That's the idea yes, but if Cambridge allocates more than 1 mark for the textual explanation, you'll need to elaborate further.You see how anally retentive Singapore JC Prelims Mark Schemes are? Cambridge may be more reasonable, but they're no less exacting.
-
Originally posted by delurach:
Is it just
1) there is an internal plane of symmetry and hence does not rotate plane polarised light, therefore is superimposable and hence is not a meso compound
2) for the other 2, they are both optically active and non-superimposable and hence exhibits optical isomerism and hence are stereoisomers
While you gave more elaboration than CB2883J, it's still not sufficiently comprehensive and exacting.As I said previously, I won't give the full model-answer, but I'll just comment on the attempted answers you guys submit, and will only comment on whether Cambridge will award you full marks (eg. assume the question provides 3 marks for the drawing, and 3 marks for the textual explanation).
Which reminds me, delurach, your earlier diagram https://i.imgur.com/a16fSCh.jpg for enantiomers with just 1 chiral C atom (ie. for other 'normal' questions), is still 'wrong' (ie. may be penalized for A level purposes), because when drawing the dash-wedge stereochemical formula for A level purposes, one of the normal (ie. neither dash nor wedge) bond must be the top bond, and the 2 normal bonds must be adjacent to each other. Your earlier drawing didn't fulfill both conditions.
-
Originally posted by UltimaOnline:
That's the idea yes, but if Cambridge allocates more than 1 mark for the textual explanation, you'll need to elaborate further.You see how anally retentive Singapore JC Prelims Mark Schemes are? Cambridge may be more reasonable, but they're no less exacting.
How else can it be elaborated? That they are mirror images and 1 rotates plane polarised light right, the other left?
-
Originally posted by delurach:
How else can it be elaborated? That they are mirror images?
Not just.I shan't give the full model-answer here, so I can't say anything more, but if you like, you (any student reading this) can attempt a more complete answer for my comment.
Or if you (all students reading this) are satisfied with your own answer, then let's move on.
No one has replied me (correctly, at least) on my other 3 BedokFunland JC questions I posted earlier in this thread. You guys can attempt those, or continue on with your 2017 Prelim Papers or TYS qns, and ask about them here.
Btw, I may not be available to help out with any more questions by tomorrow morning (ie. for the several hours before P3), so better faster ask any qns now. And if someone asks a qn in the few hours before P3, the rest of you students pls help out with the student's query, k?
-
Originally posted by UltimaOnline:
Not just.I shan't give the full model-answer here, so I can't say anything more, but if you like, you (any student reading this) can attempt a more complete answer for my comment.
Or if you (all students reading this) are satisfied with your own answer, then let's move on.
No one has replied me (correctly, at least) on my other 3 BedokFunland JC questions I posted earlier in this thread. You guys can attempt those, or continue on with your 2017 Prelim Papers or TYS qns, and ask about them here.
Btw, I may not be available to help out with any more questions by tomorrow morning (ie. for the several hours before P3), so better faster ask any qns now. And if someone asks a qn in the few hours before P3, the rest of you students pls help out with the student's query, k?
Ok i’ll give it 1 last try..
1) The molecule does not exhibit stereoisomerism as it has an internal plane of symmetry, rendering the images superimposable. By this, rotation in both clockwise and anti-clockwise direction would be cancelled out, and therefore it does not exhibit optical isomerism.
2) The other 2 molecules are mirror images of each other and do not have an internal plane of symmetry. Therefore the molecules and non-superimposable and rotation in both anti and clockwise directions will not cancel out. Hence they are optically active and are stereoisomers.
-
Hello, i’ve Got a query here! From Dunman high p3
(b) A compound X, CH2CCH3(CH2)3CaHbO, is a constitutional isomer of Geraniol. It exhibits stereoisomerism and has a particular functional group that can be tested positively by Fehling’s solution, an alkaline solution of complexed Cu2+(aq), in a redox reaction. In Fehling’s solution, the tartrate ion forms a complex with Cu2+ as shown below. (i) Suggest why the copper ion needs to be complexed for the test to work. [1]
how come only when it is complex then it wouldn’t form cu(oh)2?
-
Originally posted by delurach:
Ok i’ll give it 1 last try..
1) The molecule does not exhibit stereoisomerism as it has an internal plane of symmetry, rendering the images superimposable. By this, rotation in both clockwise and anti-clockwise direction would be cancelled out, and therefore it does not exhibit optical isomerism.
2) The other 2 molecules are mirror images of each other and do not have an internal plane of symmetry. Therefore the molecules and non-superimposable and rotation in both anti and clockwise directions will not cancel out. Hence they are optically active and are stereoisomers.
Better, but still not perfect. 2 out of 3 marks. We'll leave it as that.Besides, Cambridge is usually somewhat reasonable, lenient, and flexible. The Cambridge Mark Scheme for such questions will usually accept any x out of y possible points (where x < y), for full x marks (rather than y marks). So no worries.
-
Originally posted by delurach:
Hello, i’ve Got a query here! From Dunman high p3
(b) A compound X, CH2CCH3(CH2)3CaHbO, is a constitutional isomer of Geraniol. It exhibits stereoisomerism and has a particular functional group that can be tested positively by Fehling’s solution, an alkaline solution of complexed Cu2+(aq), in a redox reaction. In Fehling’s solution, the tartrate ion forms a complex with Cu2+ as shown below. (i) Suggest why the copper ion needs to be complexed for the test to work. [1]
how come only when it is complex then it wouldn’t form cu(oh)2?
Because when it's already coordinated with its non-H2O ligands, it's not available to form hydroxide ppt. Only metal ions that have H2O ligands can have their H2O ligands deprotonated into OH-, and thereby (subsequently generating) the hydroxide ppt. -
Just asking,when +NH3CONH3+ is produced from amide hydrolysis of phenobarbital(TYS 2012 Chem P3 Q2(e(ii)) H2),the amine produced is further hydrolysed to produce NH3,H2O and CO2?
-
Originally posted by MapPwner:
Just asking,when +NH3CONH3+ is produced from amide hydrolysis of phenobarbital(TYS 2012 Chem P3 Q2(e(ii)) H2),the amine produced is further hydrolysed to produce NH3,H2O and CO2?
Depends on whether acidic or alkaline hydrolysis is used. When in doubt, give both answers (with qualifications / specifications).